Abstract
Precise delineation of the shape of skull base meningiomas is critical for their treatment and follow-up but is often difficult using conventional imaging such as CT and MRI. We report our results with PET/CT and 2-18F-fluoro-l-tyrosine (18F-TYR), a marker of amino acid transport, as part of the yearly follow-up of irradiated patients. Methods: Eleven patients (mean age, 56.5 y) with skull base meningiomas (n = 13 lesions) previously irradiated were included. All patients received 300 MBq of 18F-TYR and were imaged after 30 min of uptake, using a dedicated PET/CT system. The images were first visually examined, and regions of interest (ROI) were then placed over the transaxial PET slice showing the highest uptake. Another ROI was placed over the normal parietal cortex. Tumor-to-cortex activity ratios were obtained by dividing the maximum pixel value in the tumor ROI by the maximum pixel value in the cortex ROI. The PET/CT images were compared with the MR images obtained as part of routine follow-up. Results: Accumulation of the tracer was higher in all meningiomas than in the surrounding tissue. The tumor-to-cortex activity ratio was 2.53 ± 0.35 (range, 1.3–6). Nonneoplastic tissue such as hyperemic cavernous sinus did not take up the radionuclide and was therefore easily distinguished from the meningioma. The 18F-TYR anomalies completely overlapped with the MR image in 54% of the tumors, extended beyond the MRI lesion in 38% of the tumors, and were smaller in 8% of the tumors. Conclusion: Meningiomas of the skull base are clearly visualized using 18F-TYR PET/CT, even after irradiation. In addition to MRI, 18F-TYR PET/CT images may contribute to the evaluation, delineation, and follow-up of these tumors.
Meningiomas are mostly extraaxial tumors originating from the arachnoid cells. They account for approximately 20% of all intracranial tumors (1). Although most often histologically benign, they may be associated with significant morbidity through encasement, displacement, and compression of nervous and vascular structures. When they are at the base of the skull, a complete resection of these tumors is not always possible, even with recent advances in neurosurgical techniques, because of the deep location and the risk of hemorrhage.
Modern radiotherapy methods, such as intensity-modulated radiotherapy and stereotactic techniques, can now conform the dose in 3 dimensions to the often complex shape of the tumor, avoiding high doses to critical structures such as the optic apparatus or the brain stem (2). When successful, these treatments stop the growth of the tumor or even reduce its volume in 14%–25% of the patients (3). However, a recurrence is observed after 5 y or more in approximately 15% of the patients (3), thus emphasizing the need for efficient follow-up.
Yearly neuroradiologic measurements performed in variable planes sometimes fail to show small increases in tumor volume before signs of clinical progression appear, such as a reduction of the visual field. Indeed, distinguishing between tumor and the healthy tissue of the skull base is difficult, even with modern imaging techniques (4). Both CT and MRI are used for the follow-up of meningiomas because of the usefulness of combining the superior soft-tissue imaging afforded by MRI with the geometric accuracy of CT. However, Pieper et al. showed that this combination is unable to demonstrate bony involvement in all cases (5). Thus, there is a need for additional methods to characterize these tumors during follow-up. The identification of tumors that have a tendency to grow at the sphenoid ridge into the orbit or the posterior fossa cannot benefit from PET with 18F-FDG, because glucose metabolism in the meningioma is most often similar to that in normal surrounding tissue.
Recently, various radioactively labeled compounds have emerged as potential tools to help characterize tumor shape and metabolism. Among those, 11C-methionine (MET), a marker of protein synthesis, has demonstrated high uptake by meningiomas (6), and the intensity of the 11C-MET uptake has been shown to correlate with the Ki67 index (7). Ligands of somatostatin receptors have also produced encouraging results (8,9).
2-18F-Fluoro-l-tyrosine (18F-TYR) was developed in the late 1980s and has shown high uptake in gliomas, although there is no report of its use in meningiomas (10). It is a substrate of the L-type amino acid transporter 1 (LAT1). Its synthesis was recently improved using a 4-step procedure with another precursor, 2-trimethylammonium-4-methoxy-benzaldehyde triflate. The synthesis is completed within 100 min, with high yields (25%–40%, corrected for decay) and high specific activity (11). The tracer is routinely available at our center, and efforts are being made to develop a fully automated procedure. Although the uptake mechanisms of 11C-MET and 18F-TYR differ, both compounds are markers of amino acid metabolism, with similar clinical results in neurooncology (12). Thanks to their longer physical half-life, as compared with that of 11C, fluorinated compounds can be used in nuclear medicine departments distant from a cyclotron.
This study aimed at evaluating the capacity of PET with 18F-TYR to discriminate meningioma from healthy tissue, especially in difficult locations such as the skull base. A second goal was to compare delineation of the tumor observed on PET with that observed on MRI.
MATERIALS AND METHODS
This study was approved by the Ethics Committee of the University Hospital and of the Faculty of Medicine of the University of Liège.
Patients
Eleven consecutive patients (10 women and 1 man) with an intracranial meningioma were included in this study. All benefited from follow-up MRI and 18F-TYR-PET. The total number of lesions was 13, because one of the patients had 3 different tumors. All 13 lesions were on the skull base. The mean age of the patients was 56.5 ± 3.0 y (range, 40–71 y). Eight of the 11 patients had undergone surgery more than 5 y before PET. A pathologic sample was obtained at the time of diagnosis in these 8 patients. All lesions were grade 1 tumors according to the World Health Organization classification. The largest diameter of the lesions measured with MRI ranged from 18 to 40 mm (mean, 27 mm).
All patients received radiotherapy, 7 shortly after neurosurgery as an adjuvant treatment and 4 when progression was observed. Two patients were treated by stereotactic radiosurgery, 7 received fractionated stereotactic radiotherapy, and 2 had conformal radiotherapy after subtotal resection or for recurrent disease. These treatments were given at a mean of 73 mo (range, 51–116 mo) before 18F-TYR PET was performed. In the patient who had multiple tumors, 2 of the lesions were not an indication for radiotherapy because they were not progressive, either clinically or radiologically.
PET
18F-TYR was synthesized according to the method of Lemaire et al. (11). All patients received 300 MBq through an indwelling catheter. PET/CT studies were performed using a Gemini Dual system (Philips), which combines a germanium oxyorthosilicate crystal–based Allegro PET scanner and a dual-slice CT scanner. A low-dose CT acquisition (130 keV; 80 mAs) was followed by a 15-min PET emission scan. The uptake time was 30 min. Images were reconstructed using a fully 3-dimensional row-action maximization-likelihood iterative algorithm, with a relaxation parameter of 0.006 and a “blob” radius of 2.5 (13). Data were corrected for scatter, randoms, attenuation, and decay.
Image Analysis
The PET and the CT images were coregistered and displayed using a Syntegra workstation (Philips). The images were first visually examined, and regions of interest (ROIs) were then placed over the transaxial slice showing the highest uptake. Another ROI was placed over the normal parietal cortex. Tumor-to-cortex activity ratios were thus obtained by dividing the maximum pixel value in the tumor ROI by the maximum pixel value in the cortex ROI. Such methodology is similar to that commonly used for 18F-FDG PET of primary brain tumors (14,15).
MRI
MRI was performed using a Magnetom Symphony 1.5-T machine (Siemens) as part of the yearly follow-up according to the following protocol: Turbo spin-echo T2-weighted axial sequences of the whole brain were acquired at 5-mm intervals. Spin-echo T1- and T2-weighted axial sequences of the skull base were acquired at 3-mm intervals, and after infusion of gadolinium-diethylenetriaminepentaacetic acid (Magnevist; Schering [0.2 mmol/kg of body weight]), spin-echo T1-weighted axial, coronal, and (if necessary) sagittal sequences were acquired with fat saturation.
Data Analysis
All imaging studies were reviewed by 2 nuclear medicine physicians and a radiologist. First, the PET/CT and MR images were analyzed independently. All lesions were described and recorded. In a second step, both datasets were reviewed side by side and the results were compared in terms of location and extent. Results for the activity ratios are expressed as mean ± SEM.
RESULTS
Patients' data and imaging results are summarized in Table 1. All lesions were clearly visible on the PET images. Tracer uptake was low in normal white matter and gray matter, but the oropharyngeal mucosa and the salivary glands showed significant uptake. All meningiomas exhibited higher accumulation of the tracer than the surrounding tissue. The average tumor-to-cortex ratio was 2.53 ± 0.35 (range, 1.3–6; n = 13).
Patient Data
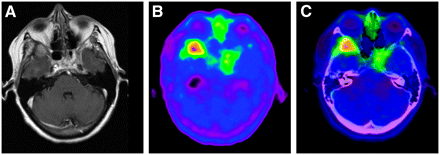
The locations of PET anomalies matched the locations of lesions on MRI in all cases. The precise delineation and extent of the tumor were similar using both modalities for 7 (54%) of 13 lesions (Fig. 1). Discrepancies between PET and MRI were observed for 6 (46%) of 13 lesions. PET lesions extended beyond MRI lesions in 5 cases (38%) (Fig. 2) and were smaller in the other case (8%) (Fig. 3).
(A) Gadolinium-enhanced T1-weighted MR image showing right retroorbital and left cavernous sinus meningioma of patient 1. (B) 18F-TYR PET image showing high uptake in both tumor locations. (C) Fused PET/CT image.
Example in which 18F-TYR PET shows crossing of midline by meningioma. (A) Spin echo T1-weighted enhanced MR image of skull base showing enlargement of right cavernous sinus. (B) Pathologic 18F-TYR uptake by tumor and physiologic uptake by nasal mucosa. (C) Fused PET and MR images.
(A) Gadolinium-enhanced T1-weighted MR image of right cavernous sinus meningioma 60 mo after radiotherapy. (B) PET image showing high 18F-TYR accumulation. (C) PET image fused with MRI showing that high-activity zone of tumor lies in sphenoidal sinus but not in hyperemic cavernous sinus (arrow).
DISCUSSION
Both CT and MRI are used in the follow-up of patients with meningioma. Bone remodeling resulting from slowly growing masses at the base of the skull is best shown by CT, but MRI is needed for better characterizing the lesion itself. The combination of both techniques, although powerful in many cases, may underestimate the extent of the tumor. In addition, neither CT nor MRI is able to differentiate hyperostosis from tumor invasion of the bone (16). Because these tumors tend to recur and progress, especially after incomplete resection, additional imaging modalities complementary to CT and MRI are needed.
Meningiomas have been shown to express somatostatin receptors both in vitro and in vivo (17,18). Somatostatin receptor scintigraphy with 111In-octreotide was thus proposed as a tool for differentiating meningiomas from other tumors and as a method for evaluating residual disease after surgery (19). However, although all tumors express somatostatin receptors, some lesions are not visible on somatostatin receptor scintigraphy (20), and even with SPECT acquisitions, the technique lacks spatial resolution. Furthermore, few data are available on the usefulness of somatostatin receptor scintigraphy in the long-term follow-up of patients with meningioma. Recently, the somatostatin analog DOTA (0)-d-Phe (1)-Tyr (3)-octreotide (DOTATOC) was labeled with 68Ga, a positron-emitting radionuclide with a physical half-life of 68 min, and showed high uptake by meningiomas, with low background activity (8). Milker-Zabel et al. reported that DOTATOC PET modified the target volume in a significant number of patients with intracranial meningiomas (21).
PET with 11C-MET has shown promising results when integrated into the radiation treatment planning of a wide variety of primary brain tumors (6,22,23). In meningiomas, 11C-MET shows a generally high but variable uptake, with indications that the intensity of the uptake is related to the proliferative activity of the tumor as measured by the Ki67 index (6,7). 11C-MET PET was recently found useful in the radiation treatment planning of meningiomas at the base of the skull, near the orbit or the cavernous sinus, in combination with CT and MRI (24). However, the use of 11C-MET is hampered by the short half-life of 11C, which, in fact, limits its application to centers with an on-site cyclotron.
To our knowledge, our study is the first to report results with TYR, which is another marker of amino acid transport, labeled with 18F. The population studied in this series was limited but homogeneous: all patients had grade I meningiomas at the base of the skull, and all were treated with irradiation. All lesions were clearly visible on the 18F-TYR scan, with an average tumor-to-background activity ratio of 2.53. Using a slightly different methodology with the cerebellum as the reference region, Nyberg et al. reported an average activity ratio of 3.68 for 11C-MET in syncytial meningiomas of the skull base (6). In another study, the same group reported an initial activity ratio of 3.5 before proton-beam radiotherapy, with a decrease to an average of 2.69 at 36 mo after irradiation (25).These values are comparable to the ratio observed in our population, which was scanned several years after completion of radiation therapy. In gliomas, we found 18F-TYR activity ratios that were in the same range as those observed with 11C-MET (26).
Although all foci of increased uptake were at the site of lesions visualized on MRI, the extent of the anomalies differed in approximately half the cases (6/13 tumors). In most cases, the discrepancy resulted from a larger extent measured on PET than on MRI. This finding somewhat contradicts the observations of Grosu et al. on the use of 11C-MET in 10 meningiomas at the base of the skull (24). In these patients, the addition of 11C-MET reduced the target volume in 40% of the cases but increased it in 20% of the cases. Several explanations are possible. First, both series were limited and any statistical considerations would be meaningless. Second, our lesions were visually analyzed, whereas ROIs were applied over the 11C-MET lesions using a methodology that was not clearly defined but was primarily manual. These differences may lead to differences in tumor volume measurements. The issue of the optimal methodology for determining target volume on metabolic images, either manually or using automated algorithms that follow activity thresholds or even more complex methods, remains open (27). Because our study did not have this purpose, and because of the lack of consensus on this question, we elected to visually analyze both datasets. Finally, although the clinical results reported for neuroimaging seem rather comparable, the uptake mechanisms of 18F-TYR and 11C-MET are quite different. 18F-TYR is handled by the L-transport system, in particular LAT1, which is bidirectional, sodium-independent, and essentially dependent on the amino acid transmembrane gradient. 11C-MET follows a complex metabolism, but uptake of 11C-MET in brain tumors reflects mainly the transport rate, essentially the L-system, but also the alanine serine cysteine and the A systems, with the additional influence of blood–brain barrier disruption (28). O-(2-18F-fluoroethyl)-l-tyrosine (18F-FET) is another fluorinated tracer with clinical results similar to those obtained with 11C-MET in gliomas (29). However, 18F-FET uptake mechanisms are different from those of other amino acid analogs. Although the mechanism is not fully understood, it most likely appears to be through another protein of system L: Although 18F-TYR is a good substrate for the LAT1 subsystem, the influx of 18F-FET through LAT1 is limited and seems rather to be handled by the L-type amino acid transporter 2 subtype (29). The clinical relevance of such a hypothesis remains unclear, however. Animal studies have shown low or no uptake of 18F-FET in inflammatory lesions (30), but false-positive results have been reported in human lesions such as abscesses (31). Little is known about the specificity of 18F-TYR, but there were no false positive results in a series of 23 patients with non–small cell lung cancer or lymphoma (32). 18F-FET PET appears able to differentiate radiation injuries from recurrent gliomas (33), although uptake in areas with postradiation changes may be moderately increased, as shown in rodent experiments (target-to-cortex activity ratio, 1.64) (34). In any case, no clinical studies have compared 18F-FET with 18F-TYR, and no clinical studies have investigated 18F-FET-PET in meningiomas. Further studies are therefore needed to evaluate whether the diagnostic performance of 18F-FET in meningiomas matches the excellent clinical results obtained in gliomas in various clinical situations. L-3-18F-Fluoro-α-methyl tyrosine, similarly to other amino acid analogs, provided high-contrast images of primary brain tumors, but only 1 case of meningioma was reported and the patient was evaluated before treatment (35). This compound has limited yields and specific activity, however. Finally, 3,4-dihydroxy-6-18F-fluoro-l-phenylalanine has also been proposed for evaluating gliomas, especially the recurrence of low-grade tumors (36), but there are no data on meningiomas.
At this stage, we can report only on the discrepancy between PET and MRI observed in 6 patients. The real extent of the tumor would be assessable only after complete surgical removal, which, of course, is not conceivable. One possible explanation for the larger tumor volume obtained with PET than with MRI may nonetheless stem from pathologic considerations. Indeed, careful histopathologic analyses have shown that, despite the benign nature of World Health Organization grade 1 meningiomas, tumoral cells may microscopically infiltrate surrounding tissue, such as vascular structures and cranial nerves (37,38). It is possible that the density of this infiltration is too low to be visualized on MR images but is sufficient to represent a focus of increased activity on PET images, thanks to the low background uptake. This reasoning is obviously hypothetical but will be prospectively tested by examining whether sites of relapse correlate with areas that are PET-positive and MRI-negative.
CONCLUSION
Persistent meningiomas of the base of the skull are clearly identified with 18F-TYR PET. In approximately half the cases, the extent of the metabolic lesions does not exactly match the MRI findings. Further studies are needed to clarify the clinical significance of this observation and to define the role of 18F-TYR in treatment planning.
Acknowledgments
We thank Profs. Achille Stevenaert and Didier Martin for referring the patients to us.
Footnotes
-
COPYRIGHT © 2007 by the Society of Nuclear Medicine, Inc.
References
- Received for publication November 15, 2006.
- Accepted for publication February 7, 2007.