Abstract
Serotonin 5-HT1A receptors have been implicated in disorders of the central nervous system and, therefore, are being studied by PET. Efforts are under way to improve in vivo stability of 5-HT1A agents currently in human use (11C-labeled N-(2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl-N-(2-pyridinyl)cyclohexanecarboxamide [11C-WAY-100635], 4-(2′-methoxyphenyl)-1-[2′-(N-2″-pyridinyl)-p-18F-fluorobenzamido]ethylpiperazine [18F-MPPF], and 18F-labeled trans-4-fluoro-N-(2-[4-(2-methoxyphenyl)piperazin-1-yl)ethyl]-N-(2-pyridyl)cyclohexanecarboxamide [18F-FCWAY]). We have synthesized N-{2-[4-(2-methoxyphenyl)piperazinyl]ethyl}-N-(2-pyridyl)-N-(4-18F-fluoromethylcyclohexane)carboxamide (18F-mefway), which contains a 18F on a primary carbon to make the compound more stable to defluorination. Methods: Radiosynthesis of 18F-mefway was performed in a single tosylate for 18F-fluoride exchange. In vitro binding studies on rat brain slices using 18F-mefway were read on a phosphor imager. Monkey PET studies were performed on a whole-body PET scanner. Results: Binding affinity (inhibitory concentration of 50% [IC50]) of mefway was 26 nmol/L and was comparable to that of WAY-100635, 23 nmol/L. Yields of 18F-mefway were 20%–30% in specific activities of 74–111 GBq/μmol at the end of synthesis. In vitro binding of 18F-mefway in the hippocampus (Hp), colliculus (Co), cortex (Ctx), and other brain regions—with limited binding in the cerebellum (Cer)—was observed, with ratios of Hp/Cer = 82.3, Co/Cer = 45.8, and Ctx/Cer = 40. Serotonin displaced 18F-mefway from various brain regions with IC50 values in the range of 169–243 nmol/L. PET studies in a rhesus monkey showed 18F-mefway binding in the fontal cortex (FC), temporal cortex (TC) including hippocampus, raphe (Rp), and other brain regions, with ratios of FC/Cer = 9.0, TC/Cer = 10, and Rp/Cer = 3.3. Plasma analysis indicated the presence of approximately 30% of 18F-mefway at 150–180 min after injection. Conclusion: The high ratios in specific brain regions such as the hippocampus suggest that 18F-mefway has potential as a PET agent for 5HT1A receptors.
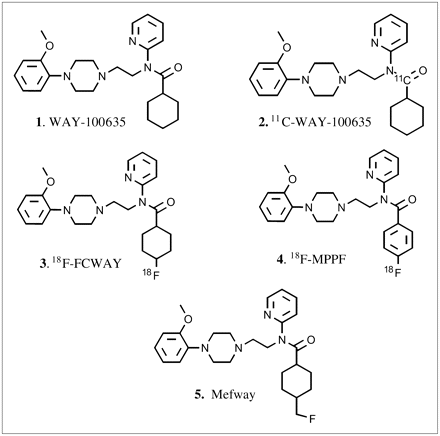
The serotonin system has been classified into 7 receptor families (5-HT1–7) that mediate the diverse actions of serotonin (1). Of the subfamilies, the 5-HT1A receptors are of interest for imaging and therapeutic applications, thus propelling development of effective PET radioligands (2,3). Several PET radioligands for 5-HT1A receptors have been developed on the basis of the antagonist WAY-100635 (N-(2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl-N-(2-pyridinyl)cyclohexanecarboxamide) (Fig. 1, 1 (4)). Three derivatives are currently used in human studies: 11C-WAY-100635, 18F-FCWAY (trans-4-18F-fluoro-N-(2-[4-(2-methoxyphenyl)piperazin-1-yl)ethyl]-N-(2-pyridyl)cyclohexanecarboxamide), and 18F-MPPF (4-(2′-methoxyphenyl)-1-[2′-(N-2″-pyridinyl)-p-18F-fluorobenzamido]ethylpiperazine) (Fig. 1, 2–4). Use of these radioligands in humans shows decreased 5-HT1A receptors in brain regions, including the raphe of depressed patients using 11C-WAY-100635 (5), decreased 5-HT1A receptor binding in the amygdala in schizophrenic patients using 11C-WAY-100635 (6), 5-HT1A receptor changes in amyotrophic lateral sclerosis (ALS) and Parkinson's disease patients using 11C-WAY-100635 (7), reduced 5-HT1A receptors in panic disorder using 18F-FCWAY (8), decreased 5-HT1A receptor binding in temporal lobe epilepsy using 18F-MPPF (9) and 18F-FCWAY (10), and loss of 5-HT1A receptors using 18F-MPPF in Alzheimer's disease (11).
Chemical structures of serotonin 5-HT1A receptor antagonists. 11C- and 18F-labeled positron-emitting derivatives of WAY-100635 (1): 11C-WAY-100635 (2), 18F-FCWAY (3), 18F-MPPF (4), and Mefway (5).
For several reasons, efforts are still under way to improve the biologic properties of currently used 5-HT1A agents for human studies (12). First, these agents lack appreciable resistance to metabolism. Specifically, cleavage of the amide bond in these radiotracers decreases plasma concentration and, consequently, little nonspecific binding is seen in the cerebellum. This causes difficulties in using the reference region method for accurate assessment of brain receptor concentrations (e.g., in 11C-WAY-100635; Fig. 1, 2 (13)). Second, with some agents (e.g., in 18F-FCWAY, Fig. 1, 3) a cleavage of the radioactive fluorine occurs, which results in low-quality images due to contamination of 18F-fluoride in the skull (14). Efforts to minimize this defluorination using cytochrome P450 2E1 isozyme inhibitor have been explored in rats with promising results (15). Third, WAY-100635 is labeled with 11C-carbon (20.4-min half-life) rather than 18F-fluorine. The longer half-life of 18F (110 min) is suitable for high-resolution PET studies for both high and low receptor concentration regions using longer scan times. Additionally, 18F allows PET centers without an in-house cyclotron to perform PET studies. Fourth, most of the imaging agents for the 5-HT1A receptor, except WAY-100635, have moderate affinity (e.g., 18F-MPPF, Fig. 1, 4 (16)). Therefore, there is a need for a more-stable, high-affinity 18F-labeled agent for the study of 5-HT1A receptors in humans. Hence, efforts are under way to improve currently used PET agents for imaging 5-HT1A receptors (12,17).
Our goal was to develop a fluorinated radiotracer for 5-HT1A receptors that would be relatively more stable to metabolism, be easily synthesized, and retain high affinity and selectivity for the 5-HT1A receptors. We hypothesized that because of significant bulk tolerance at the cyclohexyl-ring binding pocket of WAY-100635 (18), inclusion of the fluoromethyl group on this ring would produce a close structural analog of WAY-100635. Also, placing a fluorine on a primary carbon (rather than a secondary carbon as in 18F-FCWAY (19)) may enhance the compound's stability toward defluorination in vivo. We report here the following: (a) synthesis of N-{2-[4-(2-methoxyphenyl)piperazinyl]ethyl}-N-(2-pyridyl)-N-(4-fluoromethylcyclohexane)carboxamide 5 (abbreviated name: mefway, Fig. 1), (b) radiosynthesis with 18F to provide the radiolabeled analog N-{2-[4-(2-methoxyphenyl)piperazinyl]ethyl}-N-(2-pyridyl)-N-(4-18F-fluoromethylcyclohexane)carboxamide (18F-mefway), (c) in vitro binding studies on rat brain slices and the sensitivity of 18F-mefway toward serotonin in different brain regions (measuring changes in serotonin using PET and the 5-HT1A radiopharmaceuticals is an active area of study (20,21)); and (d) PET studies in a rhesus monkey to demonstrate localization of 18F-mefway to 5-HT1A receptor sites.
MATERIALS AND METHODS
All chemicals and solvents were of analytic or high-performance liquid chromatography (HPLC) grade from Aldrich Chemical Co. and Fisher Scientific. Cyclohexane-1,4-dicarboxylic acid monomethyl ester was purchased from CNH Technologies. WAY-100635 was synthesized using reported procedures (22). Electrospray mass spectra were obtained on a model 7250 mass spectrometer (Micromass LCT). Proton nuclear magnetic resonance (NMR) spectra were recorded on a Bruker OMEGA 500-MHz spectrometer. Analytic thin-layer chromatography (TLC) was performed on silica-coated plates (Baker-Flex). Chromatographic separations were performed on preparative TLC (silica gel GF, 20 × 20 cm, 2,000 μm thick; Alltech Associates Inc.) or silica gel flash column or semipreparative reverse-phase columns using Gilson HPLC systems. High-specific-activity 18F-fluoride was produced in the MC-17 cyclotron or the CTI RDS-112 cyclotron using 18O-enriched water (18O to 18F using p, n reaction). The high-specific-activity 18F-fluoride was used in subsequent reactions in automated radiosynthesis units (either a chemistry processing control unit [CPCU] or a nuclear interface 18F module). 18F radioactivity was counted in a Capintec dose calibrator, whereas low-level counting was done in a well counter (Cobra Quantum; Packard Instruments Co.). Radioactive thin-layer chromatographs were obtained by scanning in a Bioscan System 200 imaging scanner (Bioscan, Inc.). Rat brain slices were obtained on a Leica 1850 cryotome. 18F autoradiographic studies were performed by exposing tissue samples on storage phosphor screens. The apposed phosphor screens were read and analyzed by the OptiQuant acquisition and analysis program of the Cyclone Storage Phosphor System (Packard Instruments Co.). The amount of 18F-mefway was evaluated in digital light units (DLU/mm2). Monkey PET was performed using a high-resolution ECAT EXACT HR+ scanner. All animal studies were approved by the Institutional Animal Care and Use Committees of the University of California at Irvine and Wright State University.
Chemistry
N-{2-[4-(2-Methoxyphenyl)Piperazinyl]Ethyl}-N-(2-Pyridyl)-N-(4-Carboxymethylcyclohexane)Carboxamide (8).
Using reported procedures, 1-(2-methoxyphenyl)-4-[2-(2-pyridylamino)ethyl]piperazine (WAY-100634, 6, Fig. 2 (22)) (96.3 mg, 0.3 mmol) was reacted with 4-carbomethoxycyclohexane-1-carboxylic acid (7, 47.4 mg, 0.3 mmol) in the presence of benzotriazol-1-yloxytris(dimethylamino)phosphonium hexafluorophosphate (BOP; 132.0 mg, 0.3 mmol), triethylamine (125 μL), and CH3CN (1.5 mL). The mixture was stirred at room temperature for 24 h. Solvent was removed by rotary evaporation. The residue was then taken up in water (3 mL) and extracted with dichloromethane. The extracts were concentrated and purified on preparative TLC (9:1 CH2Cl2:CH3OH) to provide 8 (58 mg; 40% yield). 1H-NMR (500 MHz, CDCl3) δ ppm: 8.52–8.53 (dd, 1H), 7.76–7.79 (dt, 1H), 7.25–7.31 (m, 2H), 7.02–6.97 (m, 1H), 6.91–6.87 (m, 2H), 6.83–6.85 (m, 1H), 3.97–4.0 (t, 2H), 3.84 (s, 3H, OCH3), 3.62 (s, 3H, CO2CH3), 3.02 (br, 4H), 2.63–2.66 (m, 6H), 2.29–2.32 (m, 1H), 2.04–2.08 (m, 1H), 1.84–1.95 (m, 4H), 1.07–1.64 (m, 4H). MS, m/z, 481 (30%, [M+H]+), 503 (10%, [M+Na]+).
Synthesis scheme of mefway (5), tosylate precursor for 18F-mefway (10), and 18F-mefway (11). Reaction conditions include (i) BOP reagent, 2 eq Et3N, CH3CN, room temperature for 24 h; (ii) LiAlH4, THF, 0°C–5°C for 30 min followed by 30 min room temperature; (iii) DAST, CH2Cl2 room temperature, 24 h; (iv) p-toluenesulfonyl chloride, CH2Cl2, Et3N, room temperaure, 24 h; (v) 18F-fluoride, Kryptofix 2.2.2./K2CO3, CH3CN, 30 min. (Left inset) HPLC purification of 18F-mefway using C18 reverse-phase semipreparative column eluted with 60% acetonitrile/0.1% triethylamine at flow rate of 2.5 mL/min. 18F-Mefway had retention time of 10.5 min and specific activity of 74–111 GBq/μmol. RA = radioactivity; UV = absorbance at 254 nm.
N-{2-[4-(2-Methoxyphenyl)Piperazinyl]Ethyl}-N-(2-Pyridyl)-N-(4-Hydroxymethylcyclohexane)Carboxamide (9).
The ester (8) (48 mg; 0.1 mmol), dissolved in 1 mL of tetrahydrofuran (THF), was treated with a small amount of LiAlH4 (0.1 mL of 1 mol/L THF solution; 0.1 mmol) in an ice bath for 30 min. The mixture was allowed to stir subsequently at ambient temperature for 30 min. Excess LiAlH4 was quenched with saturated ammonium chloride, and solvents were removed by rotary evaporation. The residue was extracted with CH2Cl2 and purified by silica preparative TLC plate (9:1 CH2Cl2:CH3OH) to provide N-{2-[4-(2-methoxyphenyl)piperazinyl]ethyl}-N-(2-pyridyl)-N-(4-hydroxymethylcyclohexane)carboxamide (9) as a sticky oil (16 mg; 35% yield). 1H-NMR (500 MHz, CDCl3) δ ppm: 8.52–8.53 (dd, 1H), 7.75–7.78 (dt, 1H), 7.24–7.32 (m, 2H), 7.00–6.97 (m, 1H), 6.91–6.87 (m, 2H), 6.83–6.85 (m, 1H), 4.0 (br, 2H), 3.84 (s, 3H, OCH3), 3.39 (d, 2H, -CH2O-), 3.0 (br, 4H), 2.64 (m, 6H), 2.20 (m, 1H), 1.76–1.86 (m, 4H), 0.75–1.64 (m, 5H). MS, m/z, 453 (30%, [M+H]+).
N-{2-[4-(2-Methoxyphenyl)Piperazinyl]Ethyl}-N-(2-Pyridyl)-N-(4-Fluoromethylcyclohexane)Carboxamide (5).
The alcohol (9) (4.5 mg; 0.01 mmol) was treated with diethylaminosulfur trifluoride (DAST) (2 μL; 0.015 mmol) in CH2Cl2 (0.5 mL) while cooled in an ice-water bath. The reaction mixture was allowed to warm to ambient temperature and stirred for 24 h. The reaction mixture was washed with 10% NaHCO3 followed by water. The CH2Cl2 was dried over MgSO4, filtered, and removed by rotary evaporation. After purification by silica preparative TLC plate (9:1 CH2Cl2:CH3OH), N-{2-[4-(2-methoxyphenyl)piperazinyl]ethyl}-N-(2-pyridyl)-N-(4-fluoromethylcyclohexane)carboxamide 5 was obtained (1.8 mg; 40% yield). 1H-NMR (500 MHz, CDCl3) δ ppm: 8.53 (br, 1H), 7.86 (br, 1H), 7.6–7.7 (br, 1H), 7.33 (br, 2H), 7.20 (br, 1H), 6.99–6.93 (m, 3H), 4.4–4.3 (m, 2H), 4.2–4.1 (m, 2H), 3.93 (s, 3H, OCH3), 3.75–3.70 (dd, 2H), 3.3 (br, 4H), 3.06–3.02 (m, 6H), 1.65–1.55 (m, 4H), 0.85–1.50 (m, 5H). MS, m/z, 455 (100%, [M+H]+), 477 (8%, [M+Na]+).
N-{2-[4-(2-Methoxyphenyl)Piperazinyl]Ethyl}-N-(2-Pyridyl)-N-(4-Tosyloxymethylcyclohexane)Carboxamide (10).
The alcohol (9) (7 mg; 0.015 mmol) was reacted with p-toluenesulfonyl chloride (3.5 mg) in the presence of 2.2 μL Et3N in 0.5 mL CH2Cl2 for 24 h at room temperature. Solvent was removed by rotary evaporation. Dichloromethane was added and washed with NaHCO3 and water. The organic layer was removed, dried with MgSO4, and filtered to give N-{2-[4-(2-methoxyphenyl)piperazinyl]ethyl}-N-(2-pyridyl)-N-(4-tosyloxymethylcyclohexane)carboxamide, which was purified by silica preparative TLC plate (CH2Cl2:CH3OH, 9:1) to provide 10 (6 mg; 66% yield). 1H-NMR (500 MHz, CDCl3) δ ppm: 8.52 (d, 1H), 7.73–7.79 (m, 4H), 7.30–7.35 (m, 4H), 6.99 (br, 1H), 6.84–6.90 (m, 2H), 3.96 (br, 2H), 3.84 (s, 3H, OCH3), 3.75 (d, 2H, -CH2OSO2-), 2.98 (br, 4H), 2.61 (m, 6H), 2.44 (s, 3H, CH3), 2.10–2.20 (m, 1H), 1.85–0.80 (m, 8H). MS, m/z, 607 (20%, [M+H]+).
Radiochemistry
The radiosynthesis of 18F-mefway was performed using an automated CPCU. 18F in H218O from an MC-17 cyclotron was passed through a QMA-light Sep-Pak (Waters Corp.), preconditioned with 3 mL of K2CO3, 140 mg/mL, followed by 3 mL of anhydrous acetonitrile. The 18F trapped in the QMA-light Sep-Pak was then eluted with 1 mL Kryptofix 2.2.2./K2CO3 (360 mg/75 mg in 1 mL of water and 24 mL of acetonitrile) and transferred to the CPCU reaction vessel. The SYNTH1 program in the CPCU was used for the synthesis. This involved initial drying of the 18F-fluoride, Kryptofix 2.2.2., and K2CO3 mixture at 120°C for 10 min. Subsequently, acetonitrile (2 mL) from CPCU reagent vial 2 was added and evaporated at 120°C for 7 min to ensure dryness of the 18F-fluoride mixture. After this, the precursor, N-{2-[4-(2-methoxyphenyl)piperazinyl]ethyl}-N-(2-pyridyl)-N-(4-tosyloxymethylcyclohexane)carboxamide, 10 (1–2 mg in 0.5 mL of anhydrous acetonitrile in CPCU reagent vial 3), was added and the reaction was continued for 15–30 min at 96°C. Subsequent to the reaction, CH3OH (7 mL contained in CPCU reagent vial 4) was added to the mixture and the CH3OH contents were passed through a neutral alumina Sep-Pak (prewashed with methanol) to remove any unreacted 18F-fluoride. The collected CH3OH solution coming out of the CPCU now contained N-{2-[4-(2-methoxyphenyl)piperazinyl]ethyl}-N-(2-pyridyl)-N-(4-18F-fluoromethylcyclohexane)carboxamide, 11 (18F-mefway). The CH3OH was removed in vacuo, and the residue was taken for HPLC purification. The product was purified in a reverse-phase HPLC C18 Econosil column (250 × 10 mm; Alltech Assoc. Inc.) with 60% acetonitrile:40% water containing 0.1% triethylamine with a flow rate of 2.5 mL/min. The retention time of 18F-mefway was found to be 10.5 min (Fig. 2, HPLC chromatogram). The 18F-mefway fraction was collected into a flask and the solvent was removed in vacuo using a rotary evaporator to dryness. The radiosynthesis was accomplished in 1.5–2 h, with an overall radiochemical yield of 20%–30% decay corrected. Specific activity was measured to be 74–111 GBq/μmol.
Lipophilicity
Lipophilicity (log P) was measured to evaluate the lipid solubility of 18F-mefway by partitioning between n-octanol and 50 mmol/L Tris-HCl (pH 7.4) buffer. Log P was taken as the concentration of 18F-mefway in n-octanol over the concentration in buffer.
In Vitro Studies
In vitro binding affinities of WAY-100635 and mefway (5) to 5-HT1A receptor sites were determined from a competitive binding assay using 18F-mefway (11) as the radioligand. The brains from Sprague–Dawley rats were removed from the skull and frozen in isopentane at −20°C. Several 10-μm-thick horizontal rat brain slices were preincubated at room temperature for 15 min in 50 mmol/L Tris-HCl buffer (pH 7.4). The brain slices were then treated with increasing concentrations of WAY-100635 and mefway in the presence of 167 kBq/mL of 18F-mefway at 37°C for 1 h. After incubation, slices were washed twice with cold buffer for 1 min each, dipped in cold water, air dried, and exposed to phosphor storage screens for 24 h. To calculate the binding affinity (inhibitory concentration of 50% [IC50]) of WAY-100635 and mefway to 5-HT1A receptor sites, nonspecific binding in the cerebellum was subtracted from all samples and changes in specific binding under different concentrations of WAY-100635 and mefway were calculated. Binding affinity was computed using the KELL program (BioSoft Inc.).
For serotonin competition studies, brain slices (10 μm) were preincubated in 50 mmol/L Tris-HCl buffer (pH 7.4) for 10 min and then incubated with 130–148 kBq/mL of 18F-mefway at 37°C for 1 h. Nonspecific binding was measured in the presence of 10 μmol/L of WAY-100635. Increasing amounts of serotonin (1 nmol/L to 10 μmol/L) competed with 130–148 kBq/mL of 18F-mefway at 37°C for 1 h. After incubation, slides were washed twice (1-min washes) with ice-cold buffer. Slides were then quickly dipped in cold deionized water, air dried, and exposed to a phosphor screen for 24 h. Binding affinity (IC50) was computed as described earlier.
Monkey PET Study
A male rhesus monkey was anesthetized using ketamine (10 mg/kg) and xylazine (0.5 mg/kg) and maintained on 1%–1.5% isoflurane. Two intravenous catheters were placed, one on each arm, for administration of the radiopharmaceutical and for obtaining blood samples during the study. Vital signs of the monkey were monitored and did not show any unusual deviations from baseline values. The head of the animal was placed in the gantry of the ECAT EXACT HR+ PET scanner and positioned in place with adhesive tape. A transmission scan using a 68Ge/68Ga rod source was acquired before administration of the radiopharmaceutical to correct for tissue attenuation of the coincident radiation. A dynamic sequence of scans was acquired for 180 min after intravenous administration of 130 MBq of 18F-mefway (specific activity, 74–111 GBq/μmol). Data in the final form were expressed in units of percentage injected dose per milliliter (%ID/mL) or kBq/mL. Areas showing maximal radioligand binding in the frontal cortex, temporal cortex, dorsal raphe, and other brain regions were delineated on the images. The PET images were coregistered with an MR image template of the rhesus brain as reported previously (23). A PET image summed for a duration of 120 min was used for the PET/MRI coregistration, which was performed using the VINCI program (CPS Innovations, Inc.). Time–activity curves were obtained for all of these brain regions.
A blood analysis of the monkey PET study subsequent to administration of 18F-mefway was performed to observe levels of breakdown in the blood similar to methods described for 18F-fallypride (24). Venous whole blood (∼1 mL) was obtained at various time points (5, 10, 15, 30, 45, 60, 90, 120, 150, and 180 min) during the course of the PET study. The samples were spun in a Microfuge centrifuge (Eppendorf centrifuge 5415C) at 12,000 rpm for 5 min. Plasma (0.6 mL) was separated from each sample, and 0.1 mL was counted. The remaining 0.5 mL of plasma at each time point was combined with 0.1 mL NaHCO3, mixed well, and subsequently extracted with 0.4 mL of ethyl acetate. The ethyl acetate was separated, and 0.1 mL of ethyl acetate layer and 0.1 mL of aqueous layer were counted for analysis of 18F-mefway in blood. The aqueous layer contains hydrophilic metabolites of 18F-mefway, whereas the ethyl acetate fraction contains 18F-mefway and other lipophilic metabolites. Each of the ethyl acetate extracts was concentrated to a small volume (20 μL) and spotted (along with standard, 18F-mefway) on a large TLC plate. The plate was eluted with 9:1 CH2Cl2:CH3OH to analyze for parent 18F-mefway and metabolites. The developed TLC plate was dried, apposed overnight to a phosphor screen, and read using the Phosphor Cyclone Imager. All blood samples were counted for radioactivity in a Packard 5000 series Gamma Counter.
RESULTS
Synthesis
Production of mefway, shown in Figure 2, took place in a 3-step simplified procedure. The reaction of WAY-100634 (6) with the commercially available cyclohexane-1,4-dicarboxylic acid monomethyl ester was performed using BOP to provide N-{2-[4-(2-methoxyphenyl)piperazinyl]ethyl}-N-(2-pyridyl)-N-(4-carboxymethylcyclohexane)carboxamide (8). The yields of this coupling step were moderate (30%–40%); the acid chloride procedure as described for the synthesis of WAY-100635 may provide higher yields (22). This ester (8) was reduced with LiAlH4 more efficiently than with NaBH4, although in both cases there was significant breakdown of the amide bond. Conversion of the alcohol to the corresponding fluoro- compound (mefway) proceeded in modest yields (∼40%). The alcohol (9) was converted to the tosylate (10), with approximately >60% yield.
Radiosynthesis of 18F-Mefway
The reaction of the tosylate (10) in acetonitrile with 18F-fluoride from a MC-17 cyclotron using Kryptofix 2.2.2. and K2CO3 at 96°C for 30 min in the CPCU proceeded efficiently, to provide 18F-mefway in a single step with a radiochemical yield of 20%–30%. Semipreparative HPLC chromatographic separation of the 18F-mefway (11) product mixture gave the product radioactive peak 18F-mefway at ∼10.5 min, as seen in the HPLC chromatogram in Figure 2, with >95% radiochemical purity. This product peak was clearly separated from other mass peaks. Radiochemical yields were lower than those typically observed with tosylate-to-18F exchange reactions as previously reported (25). A large radioactive peak (seen in HPLC profile in Fig. 2) close to the dead volume remains to be identified. Stability of the product to the basic radiolabeling conditions (such as cleavage of the amide bond) needs further investigation. The specific activity of 18F-mefway was ∼74–111 GBq/μmol, which is high enough to occupy only a small fraction of the receptors and, therefore, is not expected to cause any biologic effects.
In Vitro Autoradiographic Study of 18F-Mefway
As seen in Figure 3A, in vitro autoradiography in horizontal rat brain slices displayed selective binding of 18F-mefway to the cortex, hippocampus, and colliculus, all regions rich in serotonin 5-HT1A receptors. The cerebellum showed little or no selective binding and, therefore, was used as a measure of nonspecific binding. The hippocampus gave the highest ratio of specific to nonspecific binding, 82:1, consistent with other reported WAY-100635 analogs. The colliculus and cortex, brain regions known to be less densely packed with 5-HT1A receptors, gave ratios of 46:1 and 40:1, respectively. Binding of 18F-mefway was displaced (>95% in hippocampus and cortex) in the presence of 10 μmol/L of WAY-100635 (Fig. 3A), suggesting binding of 18F-mefway to areas rich in the 5-HT1A receptor.
(A) In vitro horizontal brain slices of rat brain show binding of 18F-mefway (red = highest binding and white = lowest binding). (Left) Rat brain slice, 10 μm thick, shows brain regions. (Center) Same rat brain slice after treatment with 130–148 kBq/mL of 18F-mefway. (Right) Rat brain slice with nonspecific binding in presence of 10 μmol/L WAY-100635. (B) Horizontal rat brain slices (with dorsal hippocampus) show in vitro binding in competitive study of 18F-mefway with increasing concentrations of serotonin (5-HT): (a) 1 nmol/L; (b) 10 nmol/L; (c) 100 nmol/L; (d) 1 μmol/L; (e) 10 μmol/L). (C) (Left) Competition curves of WAY-100635 and mefway against 18F-mefway measured autoradiographically in hippocampus of rat brain slices. Inhibition constant (IC50) of WAY-100635 = 23.2 ± 2.8 nmol/L and of mefway = 25.7 ± 2.4 nmol/L. (Right) Inhibition curves of 18F-mefway binding by serotonin measured autoradiographically in different brain regions of rat brain slices shown in B. Ctx = cortex; St = striata; Hp = hippocampus; Cb = cerebellum; Col = colliculus.
Competition Study of Serotonin with 18F-Mefway
Serotonin competition was studied in increasing concentrations of 1–10 μmol/L (Fig. 3B). It was found that 10 μmol/L serotonin displaced >90% of 18F-mefway binding. Competition curves for the change in specific binding of these brain regions with increasing concentrations of serotonin are shown in Figure 3C. Inhibition constants (IC50 values) of 243.5 ± 2.0 nmol/L (colliculus), 169.4 ± 5.0 nmol/L (hippocampus), and 218.3 ± 15 nmol/L (cortex) were measured.
Binding Affinity and Lipophilicity
Binding of 18F-mefway to the 5-HT1A receptor site was inhibited by both WAY-100635 and mefway (Fig. 3C). As WAY-100635 and mefway reached micromolar concentrations, the amount of 18F-mefway bound to the 5-HT1A receptors was <5% in all brain regions. Mefway exhibited an IC50 of 25.7 ± 2.4 nmol/L, which was similar to the affinity of WAY-100535 (IC50 of 23.2 ± 2.8 nmol/L). Lipophilicity (log P) of 18F-mefway was found to be 2.62 ± 0.06. This compares well with the reported log P of 11C-WAY-100635 and 11C-desmethyl-WAY-100635 (26), suggesting that 18F-mefway would demonstrate good brain uptake.
Monkey PET Studies
Uptake of 18F-mefway in various regions was rapid and in <2 min reached levels of >0.03 %ID/mL. This is comparable to levels attained by 18F-MPPF (16) and other related WAY-100635 derivatives in monkey PET studies (12,17). After 3 h, about 6% of the initial activity in the cerebellum was still present. A number of brain regions exhibited retention of 18F-mefway and were consistent with the presence of 5-HT1A receptors. Coregistered PET/MR images showed a high degree of binding in the hippocampus as well as other regions of the temporal cortex (Fig. 4). Several other regions in the cortex exhibited a high degree of binding. The striatum and thalamus exhibited some binding, greater than that found in the cerebellum. Discrete binding was observed in the raphe, as seen in Figure 4.
Distribution of 18F-mefway in rhesus monkey brain. Coregistered MR images with summed PET images show localization of 18F-mefway. (First and second rows) Coregistered MR images and PET images, respectively, show binding in various cortical regions, including distinct hot spot (Hs) seen in red near insular cortex. (Third and fourth rows) Coregistered MR images and PET images, respectively, show hippocampus, seen in red in 3 slices, and raphe. St = striata; Hs = hot spot; Cg = cingulate; Oc = occipital cortex; Cb = cerebellum; Th = thalamus; Tc = temporal cortex; Hp = hippocampus; Rp = raphe.
Time–activity curves for the various brain regions are shown in Figure 5A. There were 4 sets of regions with different activity levels. The highest binding regions were the hippocampus and an area in the cortex (hot spot), most probably associated with the insular cortex. Clearance from this cortical region was faster than that observed in the hippocampus. The second group was composed of the temporal cortex, cingulate gyrus, frontal cortex, and occipital cortex. The third group was the striatum, thalamus, and raphe. Fourth, the lowest binding region was the cerebellum. The cerebellum, containing little or no 5-HT1A receptors, was taken as the reference region similar to the other 5-HT1A radiotracers. Ratios of the various brain regions against the cerebellum are shown in Figure 5B. The highest ratio, between 8 and 10, was found for the hippocampus and the hot spot in the cortex. These ratios decreased after plateaus around 80 min, suggesting that a 90- to 120-min PET study should be sufficient to obtain quantitative information on receptor concentration. The second group of regions, with ratios between 5 and 8, was found for the temporal cortex, frontal cortex, occipital cortex, and anterior cingulate. All ratios decreased after peaking at ∼80 min. The third group of regions—namely, the thalamus, striatum, and raphe—had ratios of 2–3.5. At the end of the 3-h scan, ∼60% of the initial activity in the hippocampus was still specifically bound and the hippocampus-to-cerebellum ratio had decreased from a peak of 9.7 to 8.4.
(A) Time–activity curve of 18F-mefway in various monkey brain regions obtained after intravenous administration of 18F-mefway. (B). Ratio plot of various brain regions (shown in A) against cerebellum. Hp = hippocampus; Hs = hot spot; Cg = cingulate; Fc = frontal cortex; Tc = temporal cortex; Oc = occipital cortex; St = striata; Rp = raphe; Th = thalamus; Cb = cerebellum.
Hydrophilic and lipophilic components were observed in the plasma during the PET study with 18F-mefway (Fig. 6A). The aqueous fraction most likely consists of the amide-hydrolysis product, 4-18F-fluoromethylcyclohexane carboxylic acid, analogous to other reported radiotracers, such as 18F-FCWAY (14). Formation of this acid seems to plateau at around 50%–60% after 50 min following injection. Lipophilic components that were extracted into ethyl acetate indicated primarily the parent, 18F-mefway (Fig. 6B), suggesting that radiolabeled 18F-mefway is likely the principal component in the brain. The standard 18F-mefway spotted on the plate was ∼80% pure. At 3 h after injection, ∼30% of 18F-mefway still remained unmetabolized in plasma. These levels are greater than those reported for some previous 5-HT1A radioligands.
(A) Blood analysis of 18F-mefway in rhesus monkey shows 18F activity in ethyl acetate and aqueous fractions. In ethyl acetate fractions, at 3 h after injection, ∼30% of 18F-mefway remained unmetabolized in plasma. (B) Thin-layer chromatographic analysis of ethyl acetate fraction of plasma obtained during PET study (0–180 min) shows presence of 18F-mefway (standard [Std] 18F-mefway shown for comparison).
DISCUSSION
Although WAY-100635 derivatives are currently being used in human studies, there is a need for improvement in their in vivo stability and imaging characteristics. Our goal was to improve on these currently used compounds by inclusion of the radioactive fluorine on a primary carbon—the fluoromethyl group of the cyclohexyl ring of WAY-100635. The fluorine atom and the amide bond in 18F-mefway have proven to be more stable. No uptake of radioactivity was seen in the skull of the monkey, suggestive of little defluorination of 18F-mefway in the PET study.
The synthesis of mefway was a 3-step procedure, and the synthesis of 18F-mefway was a 4-step procedure, starting from WAY-100634, the known intermediate. Further work, to study and improve the chemical and radiochemical yields of 18F-mefway, is ongoing. For example, we are currently evaluating the use of acid chloride coupling to provide the ester (8) to obtain higher yields compared with BOP coupling. The reaction conditions for the reduction of the ester using hydride reagents are also being optimized.
Binding affinity (IC50 values) of mefway and WAY-100635 against 18F-mefway exhibits the relative strength with which the two compounds bind to 5-HT1A receptor sites. WAY-100635 and mefway were found to have very similar binding affinity (23 vs. 26 nmol/L). This confirms our initial design of the molecule, where a similar backbone structure of the two compounds was seen. The similar affinity of 18F-mefway and WAY-100635 suggests potentially good in vitro and in vivo binding characteristics. Our experimental log P value of 2.62 ± 0.06 is within the optimal lipophilicity range of the log P value of 2.0 ± 0.5 for imaging agents, thus affirming the suitability of 18F-mefway.
The ability to measure changes in serotonin levels in the brain is an active area of investigation. Small competitive effects of drug-induced elevated serotonin levels on the binding of these radiotracers have been reported. A 10%–20% decrease in the binding of 11C-WAY-100635 (27), minor decreases in the binding of 18F-MPPF in monkey raphe (10), and some decreases in the binding of 18F-MPPF in monkeys at high serotonin concentrations have been reported (28). A decrease (27%–76%) in 18F-MPPF binding in electrically stimulated rodent raphe has been observed using microdialysis methods (29). More recent findings now suggest that the fenfluramine-induced drug effects may not be significant and could be due to the effects of blood flow (21,30).
Using microdialysis, fenfluramine (10 mg/kg) increased the concentration of serotonin to about 35–40 nmol/L compared with baseline levels of ∼5 nmol/L (20). In vitro studies of 18F-mefway showed inhibition by serotonin (IC50 = 169–244 nmol/L). At 100 nmol/L serotonin, about 30%–40% of 18F-mefway is displaced. Theoretically, therefore, we would expect to see some effect of serotonin release on 18F-mefway binding in vivo. This affinity of serotonin for 18F-mefway is similar to that measured for WAY-100635 (31). Because 11C-WAY 100635 has not been able to detect serotonin release, it remains to be shown whether 18F-mefway would be any different. It must be noted that the 18F radiolabel in 18F-mefway may allow longer imaging times to permit detection of small changes more easily compared with 11C. Therefore, 18F-mefway's ability to measure changes in brain serotonin levels may permit an understanding of alterations in concentrations of serotonin under certain biologic and physiologic states.
The uptake and retention of 18F-mefway were found to be similar to that found for 11C-WAY-100635. The kinetics approach pseudoequilibrium at around 80 min in the highly receptor-rich regions such as the hippocampus. The ratio of hippocampus to cerebellum exceeded 9.7 at this point. This selectivity of in vivo binding exceeds many of the known binding properties of 5-HT1A radiotracers. Several cortical regions also exhibited a high degree of binding consistent with the known distribution of 5-HT1A receptors. Regions such as the raphe exhibited a ratio of 3.5. These ratios compare, and even improve on, known ratios of 11C-WAY-100635, 18F-MPPF, and 18F-FCWAY (Table 1). Also, in the 18F-mefway monkey study, no defluorination products in the monkey skull were observed (as with 18F-FCWAY (2)). Binding of the 18F-mefway to other regions of the brain, such as the colliculi, and other cortical regions needs to be further analyzed. Blood analysis indicates the presence of a significant amount of the parent 18F-mefway, suggesting that cerebellum may be suitable for use in reference region analysis of the binding of 18F-mefway. Preliminary distribution volume ratios in the range of 1.8 to >7 were measured for various regions and are consistent with the findings with 11C-WAY-100635 (13). Test–retest studies are planned to further confirm the in vivo binding properties of 18F-mefway.
Comparison of Serotonin 5-HT1A Receptor PET Agents
Table 1 summarizes some of the properties of the tracers currently being used in human studies along with 18F-mefway. Brain uptake of all 4 radiotracers is comparable. The highest ratios (hippocampus/cerebellum, ∼10) were observed for 11C-WAY-100635 and 18F-mefway in monkeys. In human studies using the reference region method, the binding potential (which is a measure of receptor concentration) for hippocampus has been the highest for 11C-WAY-100635. Binding potentials with 18F-MPPF are low and consistent with the weaker affinity of MPPF for 5-HT1A receptors. The high target-to-nontarget ratios observed in the hippocampus, cortex, dorsal raphe, and other regions with 18F-mefway seem to be consistent with results obtained for 11C-WAY-100635 (32). Thus, 18F-mefway appears to be a good 18F analog of WAY-100635, which may provide greater sensitivity to measure alterations in this receptor system, compared with 18F-FCWAY and 18F-MPPF. This would be important in the diagnosis of mild cognitive impairment, Alzheimer's disease, and other disorders in which this receptor is implicated.
CONCLUSION
The new radiotracer 18F-mefway provides suitable in vitro and in vivo binding characteristics to the 5-HT1A receptor. The compound retains high binding affinity, displays optimal lipophilicity, and is radiolabeled efficiently with 18F-fluorine in a single step. High target-to-nontarget ratios in receptor-rich regions are seen both in vitro and in vivo. These improved ratios, compared with those of the currently used 18F-labeled WAY-100635 analogs, make 18F-mefway more suitable for human studies. Furthermore, the sensitivity of 18F-mefway to be displaced by serotonin suggests its value in measuring serotonin concentration changes in the living brain. Further studies are under way to demonstrate the prospects of 18F-mefway as an imaging agent for diagnosis and for potential therapy planning of 5-HT1A receptor disorders.
Acknowledgments
This research was supported by the Biological and Environmental Research Program, U.S. Department of Energy, grant DE-FG03-02ER63294, and the Undergraduate Research Opportunities Program of the University of California at Irvine. We thank Xiaohu (Shawn) Ouyang for synthesis of WAY-100635. This research was presented in part at the 52nd Annual Meeting of the Society of Nuclear Medicine, Toronto, Ontario, Canada, June 18–23, 2005.
Footnotes
-
COPYRIGHT © 2006 by the Society of Nuclear Medicine, Inc.
References
- Received for publication February 9, 2006.
- Accepted for publication June 26, 2006.