Abstract
The American College of Surgeons Oncology Group recently completed a trial evaluating the role of PET with 18F-FDG in patients with documented or suspected non–small cell lung cancer. Subjects underwent standard imaging to exclude metastatic disease before PET. Here, we report the yield of brain PET in evaluating, for potential intracranial metastases, patients who have undergone previous brain CT or MRI with negative findings. Methods: A total of 287 evaluable patients who had been registered from 22 institutions underwent whole-body 18F-FDG PET, including dedicated PET of the brain, after routine staging procedures had found no suggestion of metastatic disease. Patients were followed postoperatively for disease-free and overall survival, with a minimum follow-up of 6 mo. Patients with specific brain abnormalities identified by PET were further examined, and the findings were evaluated along with the results of CT and MRI, clinical management, and follow-up. Results: In 4 patients, PET found focal 18F-FDG uptake in the brain suggestive of metastatic disease; however, metastatic disease was excluded clinically in all 4 by negative findings on further brain imaging. All 4 patients remained alive at follow-up (mean duration, 10.5 mo; range, 6–16 mo). Conclusion: In patients with suspected or proven non–small cell lung cancer considered resectable by standard imaging, including routine preoperative contrast-enhanced CT or MRI of the brain, PET of the brain provides no additional information regarding metastatic disease.
Improvements in noninvasive staging modalities for non–small cell lung cancer (NSCLC) contribute to the accuracy of preoperative treatment decisions and prognostic estimates for patients. A major advance in the diagnosis and staging of NSCLC has been the addition of PET with the glucose analog 18F-FDG (1,2). As opposed to CT and MRI, which provide anatomic information, 18F-FDG PET provides information on the metabolic function of a potentially malignant lesion.
Discovery of distant metastatic disease generally precludes pulmonary resection in patients with NSCLC. The use of PET for the detection of distant metastases has previously been studied; reports have documented unexpected metastatic disease in 10%–20% of patients who are otherwise deemed candidates for surgical resection of NCSLC (3,4). 18F-FDG PET is an attractive modality for finding distant metastases because the whole body can be evaluated in a single imaging session.
To clarify the role of 18F-FDG PET in the staging of patients with potentially resectable NSCLC (stages I, II, and selected IIIA), the American College of Surgeons Oncology Group (ACOSOG) undertook the multiinstitutional trial Z0050. The primary objective of this trial was to evaluate the additional utility of whole-body and brain PET for staging disease in patients with documented or suspected NSCLC who had completed routine staging procedures. This study demonstrated that whole-body PET changed the management of 1 of 5 patients with potentially resectable NSCLC; the primary results of this study have been published previously (5).
As an adjunct to the primary purpose of the ACOSOG Z0050 study, the utility of PET in identifying patients with potential brain metastases was examined.
MATERIALS AND METHODS
Study Design
Patients with known or strongly suspected NSCLC that had been clinically staged through standard procedures as I, II, or IIIa and who were surgical candidates were registered in ACOSOG Z0050. Further details of study design, subject inclusion criteria and radiologic assessments, and complete 18F-FDG PET performance criteria have been described previously (5). As a component of standard staging, the study protocol required that CT or MRI of the brain be performed before and after contrast agent administration (unless use of contrast material was contraindicated).
18F-FDG PET
Before enrolling patients in the study, each participating institution was required to submit its protocol for PET along with images from 3 consecutive PET studies for evaluation and approval by the ACOSOG PET Quality Assurance Committee. Full-ring dedicated PET scanners with bismuth germanate (BGO) or sodium iodide detectors and with a manufacturer-quoted in-plane spatial resolution of less than 6 mm were used. Integrated PET/CT scanners were not used in this trial. The radiochemical purity of the 18F-FDG was required to be greater than 90%. With a dedicated BGO PET system, the 18F-FDG dose was 5.2–7.8 MBq/kg, with a minimum dose of 370 MBq. With a sodium iodide PET system, a dose of 2.6 MBq/kg was used.
PET of the body was performed first, beginning approximately 45–60 min after 18F-FDG injection. The region that was imaged extended from the upper or mid neck to the upper thigh. After body imaging, a 3-dimensional (10-min duration) or 2-dimensional (30-min duration) emission PET study of the brain was performed. Calculated attenuation correction was applied to brain emission data. The brain PET images were reconstructed by filtered backprojection with the use of a Hann filter (frequency cutoff 0.4 × Nyquist = 0.2 cycles/pixel).
An experienced nuclear medicine physician from each participating site interpreted the 18F-FDG PET images. The images were initially interpreted without knowledge of the results of previously obtained imaging studies (including brain CT or MRI) or of surgical staging procedures. The images were then reinterpreted, in standard clinical fashion, with the help of the other available conventional imaging studies (including the previously obtained CT or MRI studies of the brain). Only these “unmasked” interpretations were used in the data analysis for the study.
Follow-up
If surgical resection was performed, follow-up of the patient and the disease status was required at a minimum of 6 mo. For subjects who did not undergo surgical resection, follow-up was optional. For the subjects specifically described in this article, additional follow-up regarding vital status and disease recurrence or progression was sought from the local institution. A brain lesion reported as a probable or definite abnormality by PET was considered a false-positive result if the lesion regressed or remained unchanged at the 6-mo follow-up by imaging assessment.
Data Analysis
The results of brain CT, MRI, and PET were tabulated and compared with patient follow-up and survival as the reference standard. In addition, preoperative assessment of clinical signs and symptoms was reviewed, and vital status was determined, to confirm or disprove a PET suggestion of central nervous system (CNS) involvement. Binomial confidence intervals were calculated according to the method of Jennison and Turnbull (6).
RESULTS
From January 2000 to December 2002, 302 eligible patients were registered in Z0050 and deemed evaluable for intrathoracic disease by both imaging and operative staging. Of these 302 patients, 15 were excluded because their PET studies did not include brain imaging as specified in the protocol, leaving 287 patients who were ultimately evaluable for possible brain metastases.
Nine of the 287 evaluable patients, with negative findings on brain CT or MRI, had focal neurologic signs or symptoms suggestive of possible CNS involvement; in none of these patients did subsequent PET find evidence of intracranial metastases.
Overall, 8 patients were reported to show lesions on PET that were graded as “probably” or “definitely” abnormal. In 4 of these patients, the PET images showed focally decreased 18F-FDG uptake thought to be due to a prior stroke, surgery, or dementia. The other 4 patients showed focally increased uptake in the brain on PET that was determined by the interpreting radiologist to be “probably abnormal” and, furthermore, suggestive of metastatic disease (Table 1; Figs. 1 and 2). The studies for all of these 4 patients had been performed with BGO-crystal PET scanners. All 4 of these patients had undergone contrast-enhanced head CT as part of standard staging; all these studies were negative for malignant intracranial disease. None of these patients demonstrated focal neurologic signs on screening physical examination, and none complained of neurologic symptoms suggestive of possible CNS involvement at the time of eligibility assessment.
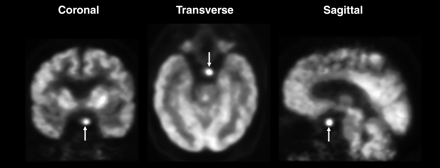
Selected brain 18F-FDG PET images of 56-y-old man (patient 1) with adenocarcinoma of right middle lobe demonstrate small focus of increased uptake (arrows) in left cerebellar tonsil. Subsequent MRI showed no evidence of metastasis. Patient underwent resection of lung cancer and was free of disease at 7-mo follow-up.
Selected brain 18F-FDG PET images of 73-y-old man (patient 2) with mixed adenocarcinoma/neuroendocrine tumor of right lower lobe demonstrate increased 18F-FDG uptake (arrows) in pituitary gland. Pituitary adenoma was considered less likely than metastasis because of intensity of this lesion. Subsequent MRI showed changes typical of pituitary adenoma but also demonstrated enhancing lesion suggestive of metastasis in right cerebellar peduncle/pons (which was not seen on PET images). Patient underwent resection of lung cancer and was free of disease at 13-mo follow-up.
Z0050 Subjects with Brain PET Findings Suggestive of Metastasis
For evaluation of their positive PET findings, 3 patients subsequently underwent contrast-enhanced brain MRI; all these studies were negative for brain metastasis. The fourth patient underwent both follow-up CT and MRI with contrast enhancement; these studies confirmed the presence of a benign pituitary adenoma that corresponded to the abnormality seen on PET, but the studies also demonstrated a possible metastatic focus involving the pons and adjacent cerebellar peduncle (for which there had been no correlate on PET). At the surgeon's discretion, 3 of these patients underwent immediate surgical exploration and were deemed surgically resectable with stage IA or IB disease. The fourth patient underwent neoadjuvant chemoradiation for stage IIIA disease, followed by successful surgical resection.
With a mean follow-up of 10.5 mo (range, 6–16 mo), none of the 4 patients with intracranial abnormality, suggestive of metastasis, on diagnostic brain PET demonstrated clinical outcomes consistent with the specific CNS abnormality identified by PET at the time of primary diagnosis. With no definite instances of true-positive 18F-FDG PET findings for cerebral metastatic disease in 287 patients, the 95% confidence interval for the yield of PET in our patient population is estimated at 0%–1.04%.
DISCUSSION
The initial staging of NSCLC is critical to the appropriate selection of patients for potentially curative surgical resection. The discovery of extrathoracic metastatic disease is a contraindication to surgery except in very specialized circumstances, such as solitary brain metastasis. The frequency of brain metastases at the time of initial diagnosis with NSCLC is approximately 10% (7). In the past, CT of the brain was recommended in addition to the neurologic work-up of patients at the time of diagnosis, to identify patients with clinically occult CNS lesions that would preclude immediate thoracic surgery (8,9). A more recent review of the literature suggests that the routine use of brain CT is not justified in early-stage NSCLC patients without neurologic signs or symptoms suggestive of intracranial involvement (10,11). For identifying brain metastases after complete resection, the use of CT has been advocated as part of an intensive follow-up regimen for patients with NSCLC (12).
Preoperative assessment and postoperative surveillance with MRI may result in earlier detection of brain metastases for patients with NSCLC. In a study of 332 patients with potentially operable NSCLC, and without clinical evidence of intracranial involvement, MRI demonstrated a tendency toward higher preoperative detection of occult brain metastases, when compared with CT (13). Because patients with isolated brain metastases may be candidates for curative surgery or radiotherapy, early identification of such lesions is critical and favors the use of more accurate modalities, such as CT or MRI, rather than PET.
Our understanding of the usefulness of 18F-FDG PET in the staging of lung cancer continues to evolve. 18F-FDG PET has been reported to detect occult extrathoracic metastases in 10%–20% of patients with disease deemed resectable by conventional methods (3,14–16). For patients with a suggestion of cerebral metastasis visualized by either CT or MRI, whole-body PET may be useful in distinguishing between patients with intracerebral lesions alone, suggesting a primary CNS process, and systemic malignancy, by identifying additional extracranial lesions to be addressed clinically (17,18).
In previous studies, PET has performed inferiorly to other modalities in the initial assessment for brain metastases, because lesions are often small and obscured by the high 18F-FDG uptake of normal cortical gray matter (19–21). The utility of routine brain 18F-FDG PET, as a component of whole-body PET, for the evaluation of CNS metastases has been questioned in several single-center studies. In a retrospective study of 40 patients with non-CNS malignancy, the findings of brain 18F-FDG PET and MRI were compared; the sensitivity and specificity of 18F-FDG PET for the detection of brain metastases were 75% and 83%, respectively (21). In a review of 20 patients with CNS metastases identified by CT or MRI, only 68% of the metastatic lesions were identified using 18F-FDG PET (20).
In another study of 273 patients undergoing evaluation for suspected malignancy, supplementary 18F-FDG PET of the brain was performed in addition to contrast-enhanced CT. 18F-FDG PET detected pathologic lesions in 6 patients (2%), but only 2 of these were unsuspected after CT at the time of PET (22). Similar results were reported more recently in a study of 1,026 patients undergoing whole-body 18F-FDG PET, including the brain, for non-CNS cancer; unsuspected skull or cerebral lesions were found in only 0.4% of the patients (23).
In the specific context of NSCLC, several studies have been published. In a retrospective study of 34 patients with potentially operable NSCLC, whole-body PET identified extrathoracic metastases in 7 patients, including 3 with confirmed CNS metastases (24). In another study, of 100 patients with newly diagnosed bronchogenic carcinoma, 78 patients underwent conventional CT or MRI of the brain in addition to whole-body and brain PET. PET correctly identified 3 of the 5 patients with confirmed brain metastases, and 1 patient was overstaged by PET but had no evidence of CNS involvement on follow-up MRI (25).
The Z0050 trial demonstrated that PET has the ability to prevent nontherapeutic thoracotomy in a high percentage of patients, even after thorough standard staging procedures. However, the detection of unsuspected metastases was much lower, only 6.3%, and there were no patients diagnosed by PET with brain metastases. Also, the study was not designed to assess the utility of PET as an initial evaluation for metastatic disease, because patients with metastases discovered by routine scans before PET were not eligible to be included in Z0050. This study did not examine the sensitivity and specificity of PET for detecting brain metastases in all patients with suspected NSCLC, because patients were screened with standard staging procedures before study registration and whole-body PET.
This study had several limitations. Patients who did not undergo immediate pulmonary resection were not followed with repeat imaging, and additional information about recurrence and survival was not required for these patients. For a complete assessment of the accuracy of PET for CNS staging, follow-up imaging with additional PET and CT or MRI would be necessary. Although a sample of study images was centrally reviewed to confirm PET image quality and protocol adherence, a full and independent review to confirm local imaging interpretation was not performed for this study. Finally, although follow-up (for survival status) was performed for the 4 patients described in this report, no specific imaging follow-up was required for abnormalities identified on initial brain PET.
In our study population, only 4 of 287 patients (1.4%) had findings on brain PET that were considered suggestive of intracranial metastasis. All these lesions were subsequently proven not to represent metastatic disease. Of importance is the fact that, on the basis of PET alone, these patients could potentially have been denied curative thoracic surgery. Certainly, further assessment is warranted when CNS abnormalities are identified on PET. Our findings in this prospective multicenter trial are consistent with those of previous retrospective single-institution studies documenting the low sensitivity of brain PET, relative to CT or MRI, for detection of metastatic disease (20,21).
CONCLUSION
From our findings, it is reasonable to conclude that there is little justification for routine imaging of the brain as part of a “whole-body” 18F-FDG PET study performed for initial staging in patients with potentially operable NSCLC who have had negative results on brain CT or MRI.
Acknowledgments
This work was supported by ACOSOG grant U10 CA 76001-09 from the National Cancer Institute.
Footnotes
-
COPYRIGHT © 2006 by the Society of Nuclear Medicine, Inc.
References
- Received for publication January 9, 2006.
- Accepted for publication July 6, 2006.