Abstract
Nuclear imaging using 18F-FDG is an established method for the noninvasive assessment of myocardial viability. Data on the value of 18F-FDG imaging in patients with diabetes mellitus are scarce. The aim of this study was to assess whether, in patients with diabetes mellitus and ischemic left ventricular (LV) dysfunction, 18F-FDG imaging can predict improvement of LV function and heart failure symptoms after coronary revascularization. Methods: A total of 130 consecutive patients with ischemic LV dysfunction who were already scheduled for surgical revascularization were studied; 34 of the patients had diabetes mellitus. All patients underwent radionuclide ventriculography to assess left ventricular ejection fraction (LVEF), resting 2-dimensional echocardiography to identify dysfunctional myocardial tissue, and dual-isotope 18F-FDG/99mTc-tetrofosmin SPECT after oral administration of acipimox. Nine to 12 mo after coronary revascularization, radionuclide ventriculography and echocardiography were repeated. An improvement in LVEF by at least 5% was considered significant. Results: 18F-FDG SPECT demonstrated that 610 (50%) of 1,212 dysfunctional segments were viable. Patients with and without diabetes mellitus had a comparable number of dysfunctional but viable segments per patient. Also, the number of patients with a substantial amount of dysfunctional but viable myocardium (≥4 viable segments) was comparable between the groups with and without diabetes mellitus. The presence of substantial viability on 18F-FDG SPECT was predictive of improvement in LVEF and heart failure symptoms postoperatively (sensitivity and specificity of 82% and 89%, respectively, in patients with diabetes and 83% and 93%, respectively, in patients without diabetes; not statistically significant). Conclusion: 18F-FDG SPECT is practical for routine assessment of myocardial viability in patients with ischemic LV dysfunction with or without diabetes mellitus. Patients with substantial myocardial viability on 18F-FDG SPECT have a high probability of improvement of LV function and symptoms after coronary revascularization, irrespective of the absence or presence of diabetes mellitus.
Diabetes mellitus is strongly associated with an increased risk for cardiac morbidity and mortality in patients with ischemic left ventricular (LV) dysfunction (1,2). Moreover, diabetes mellitus may accelerate the natural progression of myocardial dysfunction in these patients, resulting in heart failure (3). Therefore, evaluation of myocardial viability and the potential for coronary revascularization may be particularly relevant in patients with diabetes mellitus and ischemic LV dysfunction. Nuclear imaging using 18F-FDG is an established method for the noninvasive assessment of myocardial viability (4,5). However, not much is known on the prediction of functional outcome after revascularization using 18F-FDG imaging in patients with diabetes (6). This study was designed to examine whether, in patients with diabetes mellitus and ischemic LV dysfunction, 18F-FDG imaging can predict improvement of LV function and heart failure symptoms after coronary revascularization.
MATERIALS AND METHODS
Patient Population
The study population consisted of 130 consecutive patients with ischemic LV dysfunction who were already scheduled for surgical revascularization. The decision to perform revascularization was based on clinical grounds (symptoms, presence or absence of ischemia, coronary anatomy). Patients with primary cardiomyopathy or concomitant significant valvular disease were not included. All patients presented with heart failure as the predominant symptom, whereas 98 patients (75%) had accompanying angina pectoris. All patients were screened for diabetes mellitus before they were included in the study. A total of 34 patients had diabetes mellitus, defined according to the American Diabetes Association criteria (7). Of these 34 patients, 12 had diabetes mellitus type 1 (primarily due to pancreatic islet β-cell destruction) and 22 had diabetes mellitus type 2 (insulin resistance with an insulin secretory defect) (7). The remaining 96 patients did not have diabetes.
Study Protocol
The patients were prospectively studied according to a structured protocol. On being entered into the study, all patients underwent radionuclide ventriculography to assess left ventricular ejection fraction (LVEF), resting 2-dimensional echocardiography to identify dysfunctional myocardial tissue, and dual-isotope simultaneous-acquisition (DISA) SPECT, including 99mTc-tetrofosmin to evaluate perfusion and 18F-FDG imaging to assess glucose use. Nine to 12 mo after revascularization, radionuclide ventriculography and resting 2-dimensional echocardiography were repeated. The local ethics committee approved the protocol, and all patients gave informed consent.
Assessment of LVEF
LVEF was assessed by radionuclide ventriculography. A small-field-of-view γ-camera (Orbiter; Siemens) was used, oriented in a 45° left anterior oblique position with a 5°–10° caudal tilt. After injection of 99mTc-pertechnate–labeled autologous erythrocytes (550 MBq), radionuclide ventriculography was performed with the patient at rest and supine. LVEF was calculated by standard methods (Odyssey VP; Picker). An improvement in LVEF by at least 5% was considered significant.
Assessment of Regional Contractile Dysfunction
For echocardiography, a Hewlett-Packard Sonos-5500 imaging system was used, equipped with a 1.8-MHz transducer using second-harmonic imaging to optimize visualization of the endocardial border. Cine loops in 4 standard views were digitized (parasternal long and short axes, apical 2- and 4-chamber views). Two experienced readers, unaware of any other data, reviewed the pre- and postrevascularization images off-line in random order. The left ventricle was divided according to the standard 16-segment model suggested by the American Society of Echocardiography (8). Regional wall motion and systolic wall thickening were scored using a 5-point grading scale: 1 = normal, 2 = mildly hypokinetic, 3 = severely hypokinetic, 4 = akinetic, and 5 = dyskinetic. Segments with a wall motion score greater than 2 were considered dysfunctional. Improvement of segmental wall motion score after revascularization by at least 1 grade was considered significant, with the exception of improvement from dyskinesia to akinesia after revascularization.
Assessment of Perfusion and Glucose Metabolism
For optimal 18F-FDG uptake in the heart, all patients were asked to remain on a low-fat diet for the 24 h before the study. On the day of the study, the patients were allowed to have a light breakfast. Patients with diabetes mellitus were instructed to continue taking their antidiabetic medication. In all patients, venous blood samples ware taken at baseline to determine the glucose plasma concentration. In patients with a plasma glucose level greater than 9 mmol/L at baseline, 6 units of insulin (or more if considered necessary) were administered subcutaneously. One hour after intravenous injection of 99mTc-tetrofosmin (600 MBq), standard SPECT using low-energy collimators was performed to evaluate resting perfusion as described previously (9). DISA SPECT, to evaluate perfusion and myocardial glucose use, was performed on all patients after acipimox administration. Acipimox enhances myocardial 18F-FDG uptake by reducing the plasma level of free fatty acids (10–12). All patients received two 250-mg oral doses of acipimox (Byk). The first dose was given immediately before the start of standard 99mTc-tetrofosmin SPECT; the second dose was given immediately after completion of that acquisition. This schedule was chosen to ensure sufficiently low levels of serum free fatty acids at the time of 18F-FDG injection and to keep those levels low for a sufficient time during the period of 18F-FDG accumulation. Thirty minutes after standard 99mTc-tetrofosmin SPECT, the patients received a low-fat, carbohydrate-rich meal. This small meal further enhanced myocardial 18F-FDG uptake by stimulating endogenous insulin release. In all patients, venous blood sampling was repeated to determine the plasma glucose concentration. In patients with a plasma glucose level greater than 9 mmol/L, additional insulin was administered. Sixty minutes after the meal, and immediately after reassessment of plasma glucose concentration, 18F-FDG (185 MBq) was injected and, after an additional 45 min to allow cardiac 18F-FDG uptake, DISA SPECT was performed. Perfusion and metabolic imaging were performed with the patient at rest without stressors.
A triple-head γ-camera (Prism 3000XP; Philips) was used. The camera system was equipped with commercially available high-energy 511-keV collimators (13). The energies were centered on the 140-keV photon peak of 99mTc-tetrofosmin with a 15% window and on the 511-keV photon peak of 18F-FDG with a 15% window. Data were acquired over 360° (120 sectors of 3°), with the patient supine. Total imaging time was 32 min. Data were stored in a 64 × 64, 16-bit matrix. From the raw scintigraphic data, 6-mm-thick (1 pixel) transaxial slices were reconstructed by filtered backprojection using a Butterworth filter (cutoff frequency, 0.17 cycle per pixel; order, 3.5). Attenuation correction was not applied. Further reconstruction yielded standard short- and long-axis projections perpendicular to the heart axis. The 99mTc-tetrofosmin and 18F-FDG data were reconstructed simultaneously to obtain an exact alignment of the perfusion and metabolic images.
The perfusion and 18F-FDG short-axis slices were displayed in polar maps, which were normalized to the region with maximum perfusion activity (set at 100%); the polar maps were divided into 16 segments matching the echocardiographic segments (8,9). Two experienced observers evaluated the SPECT images. The segments were divided into 4 groups based on the tracer activities: 0 = normal tracer uptake (activity > 75%), 1 = mildly reduced tracer uptake (activity ≤ 75% and > 50%), 2 = moderately reduced tracer uptake (activity ≤ 50% and > 25%), and 3 = severely reduced or absent tracer uptake (activity ≤ 25%). Dysfunctional segments (identified by resting echocardiography) were subsequently evaluated for viability. Segments with normal perfusion (99mTc-tetrofosmin score of 1) and segments with a perfusion defect (score ≥ 2) but relatively increased 18F-FDG uptake (18F-FDG score > perfusion score; mismatch pattern) were considered viable. Segments with a perfusion defect and concordantly reduced 18F-FDG uptake (match pattern) were considered nonviable.
Statistical Analysis
Continuous data were expressed as mean ± SD, and percentages were rounded. Statistical analysis was performed with the BMDP statistical software package (BMDP Statistical Software Inc.). Continuous variables were compared using the Student t test for unpaired samples. Differences between proportions were compared using the χ2 test. A P value of <0.05 was considered statistically significant.
RESULTS
Patient Characteristics
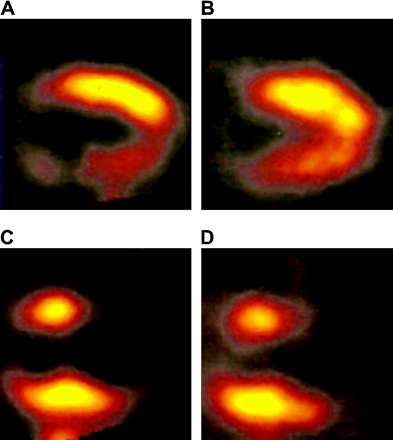
Baseline characteristics were comparable between the patients with diabetes mellitus and those without diabetes mellitus (Table 1). However, in the group of patients with diabetes mellitus, relatively more women were present (26% vs. 10%, P < 0.05). All patients presented with heart failure symptoms and had impaired LV function because of chronic coronary artery disease. The majority (123 patients, or 95%) had a previous myocardial infarction. At baseline, mean plasma glucose level was 11 ± 3 mmol/L in patients with diabetes and 5 ± 0.8 mmol/L in patients without diabetes. After subcutaneous administration of insulin (according to the SPECT protocol) and immediately before 18F-FDG injection, the mean plasma glucose level was 8 ± 3 mmol/L in patients with diabetes. Figure 1 shows examples of images obtained with DISA 99mTc-tetrofosmin/18F-FDG SPECT.
Examples of images obtained with DISA 99mTc-tetrofosmin/18F-FDG SPECT after oral administration of acipimox. Baseline 99mTc-tetrofosmin (A) and 18F-FDG (B) vertical long-axis images of patient with diabetes mellitus show perfusion defect in inferior region, with relatively preserved 18F-FDG uptake (mismatch pattern) indicating myocardial viability. Baseline 99mTc-tetrofosmin (C) and 18F-FDG (D) vertical long-axis images of patient without diabetes mellitus show large perfusion defect in apical region with absent 18F-FDG uptake (match pattern), which indicates absence of myocardial viability.
Clinical Characteristics of Patients With and Without Diabetes Mellitus
Presurgical Assessment of Myocardial Viability
Regional contractile function, as assessed by 2-dimensional echocardiography at rest, was normal in 868 segments (42%) and abnormal in 1,212 (58%). Of the 1,212 dysfunctional segments, 676 were severely hypokinetic, 521 akinetic, and 15 dyskinetic. Patients with and without diabetes mellitus had a similar number of dysfunctional segments per patient (Fig. 2). Also the number of severely hypokinetic, akinetic, and dyskinetic segments was similar in both patient subsets. Metabolic imaging with 18F-FDG SPECT showed that 610 of 1,212 (50%) dysfunctional segments were viable. Patients with and without diabetes mellitus had a comparable number of dysfunctional but viable segments per patient (Fig. 2). A substantial amount of dysfunctional but viable tissue (≥4 viable segments) was present in 14 (41%) patients with and in 52 (54%) patients without diabetes mellitus (not statistically significant).
Bar graph illustrating number of dysfunctional segments and number of viable segments, which were comparable in patients with and in patients without diabetes mellitus (neither is statistically significant). Values are expressed as mean ± SD. DM = diabetes mellitus; + = present; − = absent.
Functional Outcome After Revascularization
Functional outcome after coronary revascularization in patients with and patients without diabetes mellitus in relation to the presence or absence of substantial myocardial viability is demonstrated in Table 2. After coronary revascularization, angina pectoris symptoms (Canadian Cardiovascular Society score) improved significantly; symptoms also improved in patients without substantial myocardial viability (those with <4 viable segments). Heart failure symptoms (New York Heart Association class) improved only in patients with a substantial amount of dysfunctional but viable myocardium, irrespective of the presence or absence of diabetes mellitus. In patients without substantial myocardial viability, heart failure symptoms did not improve. Noninvasive assessment of contractile function 9–12 mo after coronary revascularization was available in 109 patients (29 with and 80 without diabetes mellitus). A total of 1,744 segments were analyzed before and after coronary revascularization, with 966 (55%) demonstrating abnormal wall motion. Improvement of wall motion after coronary revascularization occurred in 423 (84%) of 501 dysfunctional segments that were viable according to 18F-FDG SPECT. None of the dyskinetic segments improved in function. In the group of patients with a substantial amount of myocardial viability (≥4 viable segments), LVEF improved significantly after revascularization. Conversely, in the group without substantial myocardial viability, LVEF did not improve. Improvement of angina pectoris, heart failure symptoms, segmental wall motion, and LVEF was not related to the presence or absence of diabetes mellitus. The presence of a substantial amount of viable tissue (≥4 viable segments) had a comparable sensitivity and specificity for prediction of improved LVEF in patients with and without diabetes (Fig. 3).
Bar graph illustrating sensitivities and specificities of 18F-FDG SPECT for prediction of improvement of LVEF after coronary revascularization. Presence of substantial amount of viable tissue (≥4 viable segments) had good sensitivity and specificity for prediction of improvement in LVEF, which was comparable in patients with and without diabetes (neither is statistically significant). DM = diabetes mellitus; + = present; − = absent.
Functional Outcome in Patients With and Without Diabetes Mellitus According to Presence or Absence of Substantial Amount of Myocardial Viability on 18F-FDG SPECT
DISCUSSION
In patients with ischemic LV dysfunction, noninvasive assessment of myocardial viability may be relevant to selecting the appropriate management strategy (14,15). Coronary revascularization may significantly improve clinical outcome in these patients if a substantial amount of dysfunctional but viable myocardium is present. Currently, the number of patients with both diabetes mellitus and ischemic LV dysfunction is increasing rapidly (16–19). Diabetes mellitus is a recognized risk factor for coronary artery disease, and patients with diabetes mellitus have an increased risk for developing heart failure after myocardial infarction (19). Diabetes mellitus accelerates the progression of preexistent myocardial dysfunction and is associated with an increased morbidity and mortality in patients with ischemic LV dysfunction (1–3). Noninvasive assessment of myocardial viability may be particularly relevant in patients with both diabetes mellitus and ischemic LV dysfunction, because surgical revascularization in these patients is associated with a high morbidity and mortality. In the current study, 18F-FDG imaging was used to assess viability. Pooled analyses (20) have shown that 18F-FDG imaging has the highest sensitivity of all techniques for assessing viability. However, these results were obtained in patients without diabetes, and data on prediction of functional outcome using 18F-FDG imaging in patients with diabetes are scarce (6). In the present study, the impact of diabetes mellitus on prediction of clinical outcome by 18F-FDG SPECT was evaluated in 130 patients already scheduled for surgical revascularization. Metabolic imaging with 18F-FDG SPECT showed that 50% of the dysfunctional segments were viable. Patients with and without diabetes mellitus had a comparable number of dysfunctional but viable segments per patient. Also, the number of patients with a substantial amount of dysfunctional but viable myocardium (≥4 viable segments) was comparable between the groups with and without diabetes mellitus. In the patients with a substantial amount of dysfunctional but viable myocardium (≥4 viable segments), both heart failure symptoms and LVEF improved significantly, irrespective of the absence or presence of diabetes mellitus. On the other hand, in the group without substantial myocardial viability, heart failure symptoms and LVEF did not improve.
The findings obtained in the current study are in line with previous work with 18F-FDG SPECT in patients without diabetes mellitus (21,22). A recent study (22) described 47 patients with ischemic cardiomyopathy (mean LVEF, 30% ± 6%) undergoing surgical revascularization using 18F-FDG SPECT to assess viability. Regional and global function were measured before and 3–6 mo after revascularization. The presence of substantial viability (≥4 viable segments) on 18F-FDG SPECT was predictive of improvement in LVEF and heart failure symptoms postoperatively (sensitivity, 86%; specificity, 92%). The current findings extend these previous results and demonstrate the feasibility of using 18F-FDG SPECT to predict functional recovery in patients with diabetes mellitus.
Methodologic Considerations
Traditionally, 18F-FDG imaging is used in combination with PET; however, the availability of PET for cardiac imaging is limited. With the introduction of dedicated collimators for 511-keV photons, it has become possible to perform 18F-FDG imaging using SPECT, allowing 18F-FDG imaging on a larger scale (23–25). To obtain good image quality, optimization of metabolic conditions before and during 18F-FDG imaging is extremely important (26). To optimize metabolic conditions during metabolic imaging, hyperinsulinemic, euglycemic clamping can be used, which yields an excellent image quality (26,27), but this time-consuming technique is suboptimal for routine use. In daily practice, 18F-FDG imaging after oral administration of a nicotinic acid derivative (acipimox) may be preferred; this approach results in effective lowering of free fatty acids, resulting in increased cardiac glucose (and 18F-FDG) uptake. Excellent image quality with this approach has been shown, even in patients with diabetes mellitus (9), although additional insulin was sometimes needed to further increase cardiac 18F-FDG uptake and improve image quality. This was confirmed in the current study, with adequate image quality in all patients although occasional administration of insulin was needed.
Study Limitations
A potential limitation is that coronary angiography was not routinely repeated after coronary revascularization. Graft occlusion may have prevented recovery of viable segments. This may have blunted the power of 18F-FDG SPECT to predict functional improvement after coronary revascularization. Second, the SPECT protocol did not include administration of nitrates before injection of 99mTc-tetrofosmin. Nitrate administration may further enhance the detection of viable myocardium (28). Finally, in the group of patients with diabetes mellitus, relatively more women were present; this may have influenced the current results.
CONCLUSION
18F-FDG SPECT after oral administration of acipimox is practical for routine assessment of myocardial viability in patients with ischemic LV dysfunction with or without diabetes mellitus. Patients with substantial myocardial viability on 18F-FDG SPECT have a high probability of improvement of LV function and symptoms after coronary revascularization, irrespective of the absence or presence of diabetes mellitus.
References
- Received for publication June 7, 2005.
- Accepted for publication September 30, 2005.