Abstract
Tumor-induced angiogenesis can be targeted by RGD (Arg-Gly-Asp) peptides, which bind to αvβ3-receptors upregulated on angiogenic endothelial cells. RGD-containing peptides are capable of inducing apoptosis through direct activation of procaspase-3 to caspase-3 in cells. Additionally, tumor cells overexpressing somatostatin receptors can be targeted by somatostatin analogs. Radiolabeled somatostatin analogs are successfully used to image and treat such tumors via receptor-targeted scintigraphy and therapy. We combined these 2 peptides, RGD and somatostatin, to synthesize a new hybrid peptide, RGD-diethylenetriaminepentaacetic acid (DTPA)-octreotate (c(Arg-Gly-Asp-d-Tyr-Asp)-Lys(DTPA)-d-Phe-c(Cys-Tyr-d-Trp-Lys-Thr-Cys)-Thr). An earlier study showed that tumor-bearing rats had high receptor-specific uptake of RGD-111In-DTPA-octreotate in somatostatin receptor subtype 2–positive tissues and tumors. Furthermore, RGD-111In-DTPA-octreotate showed a pronounced tumoricidal effect, which is probably the result of increased apoptosis, as is shown by an increased caspase-3 activity after incubation with 111In-labeled RGD-DTPA-octreotate in comparison with the 2 monopeptides 111In-DTPA-RGD and 111In-DTPA-Tyr3-octreotate. In this study, we evaluated the biodistributions of RGD-111In-DTPA-octreotate and 125I-RGD-octreotate and investigated the caspase-3 activation of the unlabeled compound RGD-DTPA-octreotate in vitro. Methods: Biodistribution studies on tumor-bearing rats were performed with RGD-111In-DTPA-octreotate and 125I-RGD-octreotate. The apoptotic activity, by activation of caspase-3 with RGD-DTPA-octreotate and RGD-octreotate, was examined using colorimetric and immunocytochemical assays. Results: The radiolabeled compound, RGD-111In-DTPA-octreotate, showed high uptake and retention in the rats in which rat pancreatic CA20948 tumor had been implanted. A major drawback was high renal uptake. In vitro, the unlabeled peptide RGD-DTPA-octreotate induced a significant increase in caspase-3 levels in various cell lines in comparison with RGD and Tyr3-octreotate (P < 0.01). Caspase-3 activation was time dependent. To alter the elimination route, we examined the biodistribution of radioiodinated RGD-octreotate without DTPA [c(Arg-Gly-Asp-d-Tyr-Asp)-d-Phe-c(Cys-Tyr-d-Trp-Lys-Thr-Cys)-Thr], as a model of unlabeled RGD-octreotate, in tumor-bearing rats. 125I-RGD-octreotate showed a much lower renal uptake than did RGD-111In-DTPA-octreotate. Furthermore, the affinity of RGD-octreotate increased in comparison with RGD-DTPA-octreotate (values of 1.4 × 10−8 mol/L vs. 9.4 × 10−8 mol/L, respectively, for inhibitory concentration of 50%). Finally, RGD-octreotate was still able to activate caspase-3, as was indicated with immunocytochemistry. Conclusion: Because of the high renal uptake, RGD-111In-DTPA-octreotate is unsuitable for radionuclide therapy. However, the unlabeled peptides, RGD-DTPA-octreotate and RGD-octreotate, also induced an increase in caspase-3 levels, indicating the therapeutic potential of this compound. Thus, the development of hybrid molecules can become a new approach in the treatment of cancer.
Cell matrix interactions are of fundamental importance for tumor invasion and formation of metastases as well as for tumor-induced angiogenesis. Integrins, heterodimeric transmembrane glycoproteins, are composed of an α- and β-subunit and play a key role in these interactions. The main recognition site of integrins that bind to the extracellular matrix is the tripeptide sequence, Arg-Gly-Asp (RGD). The sequence was first identified in fibronectin and has since been shown to be a recognition sequence in intracellular matrix proteins such as vitronectin and fibrinogen (1,2). The vitronectin receptor, αvβ3 receptor, has been studied extensively because of its role in several biologic processes, such as angiogenesis. This receptor is expressed on various malignant human tumors and upregulated in proliferating endothelial cells.
Several compounds have been designed, on the basis of the RGD sequence, as αvβ3 antagonists. Previous studies have shown that these antagonists can regulate cell viability by inhibiting integrin binding. Disruption of the interactions between normal epithelial cells and extracellular matrix induces apoptosis and is termed anoikis (3–8). In addition, studies have shown that RGD-containing peptides may induce apoptosis independent of the association with integrin receptors by directly activating intercellular caspase-3 (9,10). These results suggest that effective and selective biologic effects on tumor tissue can be achieved using RGD peptides.
Somatostatin receptor (sst) is highly expressed on a variety of tumors, particularly those of neuroendocrine origin. Octreotide is an 8–amino acid cyclic peptide that preserves the 4–amino acid motif (Phe-Trp-Lys-Thr) that is critical for the biologic activity of somatostatin. Octreotide has a substantially longer serum half-life than does endogenous somatostatin. Radiolabeled somatostatin analogs are successfully used for the localization and staging of sst-positive tumors. Other somatostatin analogs, with a higher receptor affinity for the sst subtype 2 (sst2), are Tyr3-octreotide and Tyr3-octreotate. In the latter, the alcohol Thr(ol) at the C terminus in octreotide is replaced with the natural amino acid Thr (11). Peptide receptor radionuclide therapy can be performed using these peptide analogs radiolabeled with therapeutic radionuclides, such as the Auger electron emitter 111In and the β-emitters 90Y and 177Lu. Promising results with regard to tumor growth inhibition were shown in preclinical studies and in patient studies using 111In-diethylenetriaminepentaacetic acid (DTPA)-octreotide (12–16), 90Y-1,4,7,10-tetraazacyclododecane-N,N′,N′′,N′′′-tetraacetic acid (DOTA)-Tyr3-octreotide (17–22), and 177Lu-DOTA-Tyr3-octreotate (23,24).
Most peptide hormones are internalized after binding to specific surface receptors via invagination of the plasma membrane (25). The efficient internalization of the receptor–ligand complex into sst-positive cells forms the basis for the concept of targeted sst-mediated chemo- or radiotherapy of sst-positive tumors. Internalization brings the somatostatin analog closer to the nucleus of the cell, resulting in prolonged cellular retention and exposure to the radioactivity or cytotoxic agent (26–29). We therefore synthesized a new peptide, RGD-DTPA-octreotate [c(Arg-Gly-Asp-d-Tyr-Asp)-Lys(DTPA)-d-Phe-c(Cys-Tyr-d-Trp-Lys-Thr-Cys)-Thr]. This hybrid peptide consists of an sst-targeting peptide, Tyr3-octreotate [d-Phe-c(Cys-Tyr-d-Trp-Lys-Thr-Cys)-Thr], the chelator DTPA to enable radiolabeling, and RGD as the apoptosis-inducing peptide moiety. Recently, we reported the synthesis and characterization of this hybrid peptide in vitro and in vivo (30). RGD-DTPA-octreotate showed rapid and high-specific-activity labeling with 111In. The hybrid peptide has high affinity for both sst2-receptors and αvβ3-receptors. Furthermore, the 111In-labeled compound has a pronounced tumoricidal effect in vitro, which is better than that of the 2 monomers. The superior tumoricidal effect is probably the result of increased apoptosis, as shown by increased caspase-3 activity after incubation with RGD-DTPA-octreotate (31).
In this study, we evaluated the biodistribution of RGD-111In-DTPA-octreotate in more detail for the use of peptide receptor radionuclide therapy in rats bearing the CA20948 tumor. Because high renal uptake was observed using the radiolabeled peptide, the therapeutic effects of the unlabeled hybrid peptide in vitro in CA20948 cells, AR42J cells, and CHO cells transfected with sst2 was also evaluated. Further, a similar hybrid peptide, RGD-octreotate, without the chelator present was investigated in order to change the elimination route from the body (from renal clearance to a more hepatic clearance). The induction of caspase-3 by RGD-octreotate was also investigated.
MATERIALS AND METHODS
Peptides
The solid-phase synthesis of cyclic RGD peptides linked to the somatostatin analog Tyr3-octreotate with or without the DTPA chelator was performed using methods similar to those described by van Hagen et al. (32) for DTPA-linked RGD peptides. Synthesis was performed using a Pioneer peptide synthesizer (Applied Biosystems) and standard Fmoc solid-phase peptide synthesis methods (33). All Fmoc-protected amino acids were purchased from Novabiochem. Other peptide synthesis reagents were obtained from Applied Biosystems except as noted. Peptides were prepared on a 0.1-mmol scale with Fmoc-Thr(Otbu)-poly(ethylene glycol)-poly styrene (0.16 mmol/g loading) as the starting resin. Fmoc-protected amino acids (0.4 mmol) were activated with an equivalent amount of N-[(dimethylamino)-1H-1,2,3-triazolo[4,5-b]pyridine-1-ylmethylene]-N-methylmethan-aminium-hexaflurophosphate N-oxide (HATU) in diisopropylethylamine/N,N-dimethylformamide (DMF). Before cyclization, the allyl protecting group on the carbonyl of the aspartic acid residue at position 5 was deblocked using 4 equivalents of Pd(PPH3)4 (Sigma-Aldrich) in CHCl3/acetic acid/N-methylmorpholine (37:2:1), followed by deblocking of the N-terminal Fmoc. Cyclization was performed by activation of the free carbonyl on Asp-5 with 7-azabenzotriazol-1-yloxytris(pyrrolidino)phosphonium hexafluorophosphate in diisopropylethylamine/DMF. When peptides coupled with DTPA were prepared, the resin containing the protected, cyclized peptide was removed from the instrument, and the 4-methyltrityl protecting group on the ε-amino group of lysine-6 was removed by treatment with 5% trifluoroacetic acid/5% triisopropylsilane/90% dichloromethane (2 × 30 min). The resin was washed with dichloromethane and tetrahydrofuran before suspension in DMF (5 mL) containing 0.4 mmol of diisopropylethylamine. In a separate vessel, tri-t-butyl DTPA was dissolved in DMF containing HATU (0.4 mmol in 2.0 mL). After 1 h of mixing, the activated DTPA derivative was added to the suspended resin. The reaction was continued overnight before washing of the resin with DMF and tetrahydrofuran. Cleavage and deprotection were finally accomplished using 85% trifluoroacetic acid/5% thioanisole/5% phenol/5% water (4–6 h). The crude peptide was isolated by precipitation with t-butyl methyl ether (Sigma-Aldrich), lyophilized, and purified by reverse-phase high-performance liquid chromatography (HPLC) on a Vydac C-18 column using an acetonitrile/water gradient containing 0.1% trifluoroacetic acid (solvent A, 0.1% trifluoroacetic acid in water; solvent B, 0.1% trifluoroacetic acid/90% CH3CN in water). After loading of the peptide, column flow (10 mL/min) was isocratic at 95% A/5% B for 2.0 min before ramping to 30% A/70% B over 15 min. Molecular weight was determined by mass spectrometry using electrospray mode.
The amide cyclized RGD analog [c(Arg-Gly-Asp-dPhe-Val)] was obtained from Bachem. The peptides DTPA-RGD [c(Arg-Gly-dTyr-Lys(ε-DTPA)] and DTPA-Tyr3-octreotate [DTPA-dPhe-c(Cys-Tyr-dTrp-Lys-Thr-Cys)-Thr] were synthesized as described previously (11,32). Octreotide was supplied by Novartis.
Radiolabeling
111InCl3 was obtained from Mallinckrodt Medical BV. DTPA-Tyr3-octreotate and RGD-DTPA-Tyr3-octreotate were labeled with 111InCl3 as described previously (34). Peptides with more than 99% labeling efficiency and more than 90% radiochemical purity were used. 125I was obtained from Pharmacia. RGD-octreotate was labeled with 125I, and HPLC was subsequently performed as described earlier (35).
Cell Culture
CA20948 cells were grown in Dulbecco's modified Eagle's medium (Gibco BRL) supplemented with 10% fetal bovine serum, 2 mmol of glutamine per liter, 1 mmol of sodium pyruvate per liter, 0.1 mg of Fungizone (E.R. Squibb) per liter, and 50 IU of penicillin/streptomycin per milliliter. Rat AR42J cells were routinely maintained in F12K medium (Mediatech, Inc.) supplemented with 10% fetal calf serum, 50 μg of gentamycin per milliliter, and 2 mmol of l-glutamine per liter. Chinese hamster ovary (CHO) cells transfected with sst2 (donated by Dr. Joseph E. Bugaj) were grown in RPMI-1640 medium (Gibco BRL) supplemented with 5% fetal bovine serum, 2 mmol of glutamine per liter, 1 mmol of sodium pyruvate per liter, 0.1 mg of Fungizone per liter, 50 IU of penicillin/streptomycin per milliliter, and 1/1,000 (v/v) gentamycin (Gibco BRL). All cell lines were cultured at 37°C in 5% CO2.
Biodistribution Studies
All animal studies were conducted in compliance with the Animal Welfare Committee requirements of our institution and with generally accepted guidelines governing such work. The rat pancreatic CA20948 tumor was grown in the right flank of male Lewis rats weighing 80–120 g (Harlan). The rats received a subcutaneous injection of 106 cells from crude tumor tissue or cell culture. Experiments were initiated 15 d after inoculation. For biodistribution studies, the rats received a 3-MBq (0.5 μg) injection of RGD-111In-DTPA-octreotate into the dorsal vein of the penis. For determination of nonspecific binding of the radiopharmaceutical, some rats were coinjected intravenously with an excess of 500 μg of octreotide, RGD, or octreotide plus RGD. At 1, 4, 24, 48, 72, or 96 h after injection, rats were sacrificed. Radioactivity in tissue samples was determined using a 1282 CompuGamma system (LKB). All groups contained at least 3 rats; data are expressed as mean ± SD.
In experiments using 125I-labeled RGD-octreotate, rats received a 0.25-MBq (0.5 μg) injection of 125I-RGD-octreotate into the dorsal vein of the penis. Because HPLC purification of the iodinated peptide yielded 2 peaks, 2 separate groups of rats were used to evaluate the biodistribution of each peak. At 1 or 2 h after injection, rats were sacrificed. Radioactivity in tissue samples was determined as indicated above. All groups contained at least 3 rats; data are expressed as mean ± SD.
Caspase-3 Assay
Cell extracts were prepared and caspase-3 activity determined according to the package insert in the caspase-3 cellular activity kit of the QuantiZyme assay system (BIOMOL). The assay is based on enzymatic cleavage of the chromophore, p-nitroanilide (pNA), from the substrate NH2-Asp-Glu-Val-Asp-OH-pNA, which is measured by reading the absorbance (with a microplate reader; Bio-Rad) of the samples at a 405-nm wavelength.
Cells were treated with RGD, Tyr3-octreotate, or RGD-(DTPA)octreotate for 3–48 h. After exposure to trypsin, cells were harvested and then lysed with lysis buffer provided in the kit.
Immunocytochemistry
For apoptosis activation studies, AR42J cells were plated into 24-well plates (Corning) at approximately 0.5 × 106 cells per well. Cells were cultured for 18 h before use. Before treatments, cells were washed with phosphate-buffered saline (PBS), treated with 0.25 mL of medium containing indicated peptides, and subsequently fixed for 30 min at room temperature with 0.25 mL of 2% formalin in PBS. Wells were washed 3 times for 5 min with PBS and then made permeable in 0.25 mL of 1% triton X-100 for 10 min at room temperature. After a blocking treatment with 0.25 mL of 3% bovine serum albumin (BSA) in PBS, cells were incubated overnight with gentle agitation with primary antibodies using a 3% BSA/PBS solution containing a 1/200 dilution of rabbit anticleaved caspase-3 (Cell Signaling Technologies Inc.) and a 1/300 dilution of mouse anti β-tubulin. Cells were subsequently washed 3 times with PBS and then reacted for 1 h with a 3% BSA/PBS solution containing a 1/1,000 dilution of Alexa Fluor 594 goat antirabbit antibody and a 1/750 dilution of Alexa Fluor 488 goat antimouse antibody (Molecular Probes Inc.). Cells were washed 3 times with PBS. Fluorescence was visualized using a Diaphot inverted microscope (Nikon) equipped with epifluorescence illumination and appropriate filters. Digital images were captured using an S1Pro digital camera (Fuji), and composite images were prepared using Photoshop 5.0 (Adobe).
Autoradiography
The receptor affinity of RGD-(DTPA)-octreotate and RGD-octreotate was determined by autoradiography of brain (sst2-positive tissue) sections. Brain tissue was embedded in TissueTek (Saqura) and processed for cryosectioning. Tissue sections (10 μm) were mounted on glass slides and stored at −20°C for at least 1 d to improve adhesion of the tissue to the slide. Sections were air dried; preincubated in 170 mmol of Tris-HCl buffer per liter, pH 7.6, for 10 min at room temperature; and then incubated for 60 min at room temperature with 10−10 mol of 111In-DTPA-Tyr3-octreotate per liter. Displacement experiments were performed on adjacent sections using increasing concentrations of Tyr3-octreotate, RGD-(DTPA)-octreotate, and RGD-octreotate (range, 10−10–10−6 mol/L) in incubation buffer (170 mmol of Tris-HCl buffer per liter, pH 7.6, containing 1% (w/v) BSA, 1 mg of bacitracin, and 5 mmol of MgCl2 per liter). After incubation, the sections were washed twice for 5 min in cold incubation buffer including 0.25% BSA, in buffer alone, and in cold water purified by the Milli-Q system (Millipore). Finally, the sections were dried quickly. The sections were exposed to phosphor imaging screens (Packard Instruments Co.) in x-ray cassettes. The screens were analyzed using a Cyclone phosphor imager and a computer-assisted OptiQuant 03.00 image-processing system (Packard Instruments Co.). For each of the tested compounds, complete displacement experiments were performed using increasing concentrations of unlabeled compound ranging from 0.1 to 1,000 nmol/L. Inhibitory concentration of 50% (IC50) was calculated from the competitive binding curves using Prism software (GraphPad).
Statistical Analysis
Statistical and multiple-comparison analyses were performed within each group using ANOVA and the Bonferroni correction on Prism software.
RESULTS
RGD-DTPA-Octreotate
Biodistribution.
The biodistribution of RGD-(111In-DTPA)-octreotate was determined at 24 after injection in tumor-bearing rats (Table 1). The uptake of RGD-(111In-DTPA)-octreotate in sst2-positive tissues—for example, pancreas and adrenals—and in tumor was found to be receptor specific because uptake was substantially reduced when RGD-(111In-DTPA)-octreotate was coinjected with 500 μg of octreotide (P < 0.05). In liver (P < 0.001) and tumor, decreased uptake was found when RGD-(111In-DTPA)-octreotate was coinjected with 500 μg of RGD, indicating receptor-specific uptake in these αvβ3-positive organs, although less pronounced than in sst2-positive tissues. When RGD-(111In)-DTPA-octreotate was coinjected with an excess of octreotide plus RGD, a clear reduction (P < 0.05) was seen in both sst2- and αvβ3-positive tissues and in tumor.
Tissue and Tumor Uptake of RGD-(111In-DTPA)-Octreotate With or Without Coinjection of Octreotide, RGD, or Octreotide plus RGD
Retention of RGD-(111In)-DTPA-octreotate in tissues and tumor as a function of time was also investigated. Table 2 shows that there was high retention of radioactivity in tumor after RGD-(111In-DTPA)-octreotate injection at all time points. Because of the high renal uptake, we further investigated the therapeutic effects of the unlabeled hybrid peptide.
Tissue and Tumor Uptake of RGD-(111In-DTPA)- Octreotate (0.5 μg/3 MBq)
Caspase-3 Activity.
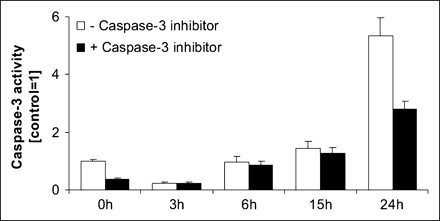
Caspase-3 activity in CA20948 cells was determined after 48 h of incubation with the peptides RGD, Tyr3-octreotate, and RGD-DTPA-octreotate at a concentration of 10−6 mol/L (Fig. 1). The highest caspase-3 activity was seen in extracts from cells incubated with RGD-DTPA-octreotate (P < 0.01). In control experiments, the 2 monopeptides, RGD and Tyr3-octreotate, induced almost no caspase-3 activation (Fig. 1). The time-dependent response of caspase-3 activation in cells that were incubated for various intervals with RGD-DTPA-octreotate is shown in Figure 2. The highest caspase-3 activity was seen after 24 h (P < 0.001 vs. control).
Caspase-3 activity in CA20948 cells after 48 h of incubation with a 10−6 mol/L concentration of RGD, Tyr3-octreotate, or RGD-DTPA-octreotate. Control received only incubation medium. Caspase-3 activity was measured in extracts prepared from cells after 48 h of incubation with the indicated peptide (white bars) or cotreated with the indicated peptide and with caspase-3 inhibitor, Ac-DEVD-CHO (black bars). Experiments were performed in triplicate, and each sample was measured in triplicate. Bars represent mean ± SEM.
Time course of caspase-3 activity in CA20948 cells treated with RGD-DTPA-octreotate. Caspase-3 activity was measured in extracts prepared from cells incubated for the indicated period with peptide (white bars) or cotreated with peptide and with caspase-3 inhibitor, Ac-DEVD-CHO (black bars). Experiments were performed in triplicate, and each sample was measured in triplicate. Bars represent mean ± SEM.
We further investigated whether this phenomenon was also found in other cell lines. We studied caspase-3 activity after incubation intervals of 6 and 24 h with the peptide RGD-DTPA-octreotate in the sst2-positive cell line AR42J and the CHO cell line transfected with sst2. As is shown in Figure 3, both cell lines had a significant increase in caspase-3 activity after 24 h of incubation with a 10−6 mol/L concentration of RGD-DTPA-octreotate (AR42J, P < 0.01; CHO sst2+, P < 0.001). After 6 h of incubation, the caspase-3 activity was at the same level as the control.
Caspase-3 activity in other cell lines after treatment with a 10−6 mol/L concentration of RGD-DTPA-octreotate [c(Arg-Gly-Asp-d-Tyr-Asp)-Lys(DTPA)-d-Phe-c(Cys-Tyr-d-Trp-Lys-Thr-Cys)-Thr] for various times: AR42J cells (A) and CHO sst2 receptor-positive cells (B). Caspase-3 activity was measured directly after each incubation period. Extracts were prepared from cells incubated with peptide (white bars) or cotreated with peptide and with caspase-3 inhibitor, Ac-DEVD-CHO (black bars). Experiments were performed in triplicate, and each sample was measured in triplicate. Bars represent mean ± SEM.
RGD-Octreotate
Because of the apoptosis-inducing activity of RGD-DTPA-octreotate observed in in vitro experiments and because of the undesirable renal uptake of RGD-111In-DTPA-octreotate, we synthesized RGD-octreotate (c(Arg-Gly-Asp-d-Tyr-Asp)-Lys-d-Phe-c(Cys-Tyr-d-Trp-Lys-Thr-Cys)-Thr) without the chelator DTPA present, to reduce renal uptake of this hybrid peptide. We first determined the affinity to the sst2 by autoradiography displacement experiments performed using brain sections.
The IC50 value of Tyr3-octreotate was 8.5 × 10−9 mol/L as calculated from data shown in Figure 4. The affinity of RGD-DTPA-octreotate and RGD-octreotate to the sst2 was somewhat lower than that of Tyr3-octreotate itself, although the affinity of RGD-octreotate (IC50 = 1.4 × 10−8 mol/L) to the sst2 was higher than that of RGD-DTPA-octreotate (9.4 × 10−8 mol/L).
Displacement curves of 111In-DOTA-Tyr3-octreotate in rat brain sections. Sections were incubated with 111In-DOTA-Tyr3-octreotate (10−10 mol/L) analog and increasing concentrations of unlabeled Tyr3-octreotate (▪), RGD-octreotate (▴), or RGD-DTPA-octreotate (•). Each point represents mean of 3 experiments of 4 sections each. Binding of a 10−10 mol/L concentration of 111In-labeled Tyr3-octreotate was considered 100%.
Biodistribution Studies.
To study the biodistribution of RGD-octreotate without the chelator (DTPA) present, we radioiodinated RGD-octreotate. HPLC analysis showed 2 peaks (Fig. 5)—a finding that was anticipated because this peptide has 2 tyrosine residues. Biodistribution studies on tumor-bearing rats using the peaks together (data not shown) and the peaks separated resulted in comparable pharmacokinetics (Table 3). Renal uptake of 125I-RGD-octreotate reached a maximum of 4.2% of the injected dose per gram at 1 h after injection, a value that is significantly lower (P < 0.001) than that for RGD-111In-DTPA-octreotate (Table 2). In contrast, liver uptake after injection of 125I-RGD-octreotate increased somewhat in comparison with liver uptake after injection of RGD-111In-DTPA-octreotate (P < 0.05).
Elution pattern of 125I-RGD-octreotate on C18 reverse-phase HPLC.
Tissue and Tumor Uptake (Peaks 1 and 2) of 125I-RGD-Octreotate
Caspase-3.
In the AR42J cells, we visualized the activation of caspase-3 by RGD-octreotate using immunocytochemistry. Figure 6, which shows untreated AR42J cells, AR42J cells treated with 0.002 mmol of RGD-octreotate per liter, and AR42J cells treated with 0.1 mmol of RGD-octreotate per liter, indicates that caspase-3 cleavage was higher in cells treated with either concentration of RGD-octreotate than in untreated cells. Thus, RGD-octreotate also is able to activate caspase-3 in the cell, thereby inducing apoptosis.
Caspase-3 activity in AR42J cells after treatment with RGD-octreotate [c(Arg-Gly-Asp-d-Tyr-Asp)-Lys-d-Phe-c(Cys-Tyr-d-Trp-Lys-Thr-Cys)-Thr]: untreated AR42J cells (A), 2 × 10−6 mol of RGD-octreotate per liter (B), and 1 × 10−4 mol of RGD-octreotate per liter (C). Green fluorescence is tubulin staining, and red fluorescence (arrows) is cleaved caspase-3 staining.
DISCUSSION
Angiogenesis is a complex process that normally occurs in adults only under specific conditions such as wound healing and inflammation (1). However, angiogenesis is also essential for the growth of tumors. The integrin αvβ3 receptor has a well-characterized involvement in angiogenesis (36,37) and tumor invasiveness (36,38) that has been demonstrated using αvβ3-specific antagonists, such as monoclonal antibodies and RGD peptides, which are able to block or interfere with these processes (3,4). Another important finding is that soluble RGD peptides are able to induce apoptosis by activation of cytoplasmic procaspase-3 (10). This direct activation of caspase could explain the apoptotic activity of RGD peptides.
Radiolabeled somatostatin analogs are used for the localization and treatment of sst-positive tumors, mostly of neuroendocrine origin. A large variety of radiolabeled somatostatin derivatives has been prepared for radionuclide therapy and visualization of these tumors and metastases, and some are in various stages of preclinical and clinical investigation. 90Y-DOTA-Tyr3-octreotide (OctreoTher; Novartis) is an example of a somatostatin analog radiolabeled with the high-energy β-emitter 90Y. More recent is the peptide 177Lu-DOTA-Tyr3-octreotate, which is a further improved targeted radiopharmaceutical because of its high rate of uptake and increased retention in sst2-positive tumors (23,24,39). It was proposed that by creating a hybrid RGD-DTPA-somatostatin peptide, the radiotherapeutic efficacy of the DTPA-somatostatin analog could be increased through the induction of apoptosis via the RGD peptide.
A previous study (30) showed that this hybrid peptide had a high uptake in sst2-positive and αvβ3-positive rat pancreatic CA20948 tumor cells. Internalization occurred mainly via sst2-receptors. Biodistribution studies of RGD-111In-DTPA-octreotate in tumor-bearing rats showed that the compound had high tumor uptake and retention of radioactivity (30). From these in vitro and in vivo studies, one can conclude that Tyr3-octreotate can serve as a carrier for RGD internalization. In vitro studies (31) showed that RGD-111In-DTPA-octreotate had a more pronounced tumoricidal effect than did 111In-DTPA-RGD or 111In-DTPA-Tyr3-octreotate in a colony-forming assay. The superior tumoricidal effect was probably the result of increased apoptosis, as shown by increased caspase-3 activity after incubation with RGD-DTPA-octreotate (31).
The biodistribution studies reported here showed high, receptor-specific tumor uptake and long-term retention of RGD-111In-DTPA-octreotate in tumor-bearing rats (Tables 1 and 2). When an excess of octreotide, RGD, or RGD plus octreotide was coinjected, uptake in various sst2- and αvβ3-receptor–positive organs was significantly reduced. The reduction in, for example, the tumor was most pronounced when the 2 peptides were coinjected (P < 0.05). The uptake of RGD-111In-DTPA-octreotate occurred via both receptors but probably was mainly via the sst2 receptor. Unfortunately, RGD-111In-DTPA-octreotate has a high renal uptake, limiting the therapeutic dose that can be administered, because the kidneys are the critical organs in peptide receptor radionuclide therapy using radiolabeled somatostatin analogs. As an alternative approach, the therapeutic effects of the unlabeled hybrid peptide RGD-DTPA-octreotate were also investigated.
The highest levels of caspase-3 activity were found in CA20948 cells after incubation with this hybrid peptide, RGD-DTPA-octreotate, in comparison with the 2 monopeptides, RGD and Tyr3-octreotate. Caspase-3 activity after incubation with RGD-DTPA-octreotate was time dependent. The highest caspase-3 activity was found after an incubation period of 24 h. The caspase-3 activity was lower after 48 h than after 24 h (data not shown). In 2 other cell lines (AR42J and CHO transfected with sst2), caspase-3 activation was also investigated after incubation with unlabeled RGD-DTPA-octreotate. Here, a significant increase in caspase-3 levels was observed, and again the highest levels were found after 24-h treatments. The caspase-3 levels were dependent on the cell line used, and the highest levels were found in the CHO sst2-positive cell line. A recent study (40) on HUVEC (human umbilical vein endothelial cells) also found an increase in caspase-3 activation after 24 h of incubation with an RGD peptide. That study found that an increase in caspase-8 and -9 levels had already occurred after 4 h of incubation (40), supporting the hypothesis that RGD peptides might directly trigger the caspase cascade at an early level.
To get an idea of the biodistribution of unlabeled RGD-octreotate without the chelator (DTPA) present, we radioiodinated RGD-octreotate. On HPLC analysis, 2 peaks were detected and isolated. Because 2 tyrosine residues are present in this molecule, one in the RGD part and one in the octreotate part, 125I can label in various ways. Biodistribution studies were performed on tumor-bearing rats using the peaks together (data not shown) and the peaks separated, and the results were comparable. The biodistribution of 125I-RGD-octreotate (Table 3) showed indeed a much lower renal uptake and a somewhat higher liver uptake than did that of RGD-111In-DTPA-octreotate. With the radioiodinated RGD-octreotate, shorter intervals were taken because release from the cells was more rapid for 125I than for the radiometal 111In. Finally, immunocytochemistry showed that RGD-octreotate was still able to activate caspase-3.
CONCLUSION
Because of the high renal uptake of RGD-111In-DTPA-octreotate, it seems unlikely that this peptide will be useful for radionuclide therapy. Conceivably, to enhance radionuclide therapy with somatostatin analogs, the unlabeled hybrid peptide could be administered after or during therapy without radiotoxicity to nontarget tissues.
Acknowledgments
We thank Renate Okker and Magda Bijster for their technical assistance with the experiments. This publication was supported in part by grant 1R43 CA101279-01 from the National Cancer Institute. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
References
- Received for publication May 17, 2005.
- Accepted for publication August 23, 2005.