Abstract
We evaluated serial changes in cardiac sympathetic nerve distribution using 123I-metaiodobenzylguanidine (123I-MIBG) after the Maze procedure. The Maze procedure, in which multiple incisions are made in the atrium, has been concomitantly performed with mitral valve (MV) surgery in an attempt to eliminate atrial fibrillation (AF). Although attenuation of the sinoatrial node response to exercise and a reduction of left ventricular function (left ventricular ejection fraction [LVEF]) in early stages after the Maze procedure have been suggested, factors leading to these changes have not been clarified. Methods: Thirteen patients with MV disease were enrolled in this study. Six of them had undergone MV surgery and the Maze procedure (Maze+), and 7 had undergone MV surgery without the Maze procedure (Maze−). All patients underwent cardiac 123I-MIBG imaging preoperatively and 10 d and 1 y after surgery to assess 123I-MIBG uptake (heart-to-mediastinum count ratio of early planar images [H/M]) and the washout rate (WR). Radionuclide ventriculography was also performed to calculate LVEF 3 d after each 123I-MIBG imaging. Results: The LVEF of the Maze+ group significantly decreased 10 d after surgery (44.2 ± 4.8; mean ± SD) compared with that before surgery (60.3 ± 6.9; P < 0.05) and significantly increased at 1 y (65.2 ± 2.9) compared with that at 10 d (P < 0.05). In the Maze− group, there was no significant change 10 d (53.0 ± 12.3) and 1 y (58.6 ± 4.8) after surgery compared with that before surgery (60.4 ± 4.6) (P = not significant, each). In the Maze+ group, the H/M (1.51 ± 0.18) was significantly lower at 10 d after than that at the preoperative stage (1.90 ± 0.25; P < 0.05) but significantly recovered at 1 y (2.23 ± 0.18; P < 0.05) with a similar transient increase in the WR (36.7% ± 6.1% at preoperative stage; 46.9% ± 3.4% at 10 d; 39.9% ± 6.5% at 1 y; P < 0.05, each). On the other hand, the Maze− group did not show a significant change in the H/M (1.94 ± 0.32, 2.06 ± 0.18, and 2.13 ± 0.17, respectively; P = not significant, each) but did exhibit a significant decrease in the WR (40.4% ± 5.1%, 37.0% ± 5.1%, and 32.9% ± 2.5%, respectively; P < 0.05, each). Changes in the H/M of both groups significantly correlated with the change in LVEF (r = 0.82; P < 0.05), and the WR showed a significant inverse correlation with changes in the LVEF (r = −0.81; P < 0.05). Conclusion: Cardiac sympathetic nerves were denervated at early stage and reinnervated at late stage after the Maze procedure. Such adrenergic nerve changes may be correlated, at least in part, with changes in left ventricular function after this procedure.
Atrial fibrillation (AF) is generated by the mechanism of reentry (1,2). The Maze procedure, in which multiple incisions are made in the atrium, has been developed and creates an electrical maze to prevent reentry in the atria and restore atrioventricular synchrotony (3).
AF is present in 40%–60% of patients undergoing mitral valve (MV) surgery (4,5). Recently, the Maze procedure has been concomitantly performed with MV surgery in an attempt to eliminate AF (3,6,7). The effectiveness of this procedure for recovery of the sinus rhythm and to reduce mortality after MV surgery has been shown, but some studies have reported a temporary attenuation in exercise capacity, chronotropic responsiveness, and left ventricular function in patients after the Maze procedure (8,9). None of these studies, however, used methods that could identify evidence of denervation and reinnervation.
123I-Metaiodobenzylguanidine (123I-MIBG), a norepinephrine analog, accumulates in the endings of sympathetic nerves through the uptake-1 mechanism and can be used to delineate cardiac sympathetic nerve distribution and function (10). Previous studies have demonstrated the correlation between changes in 123I-MIBG uptake and sympathetic neural effects on left ventricular function through heart transplantation, the arterial switch operation, and surgical repair of ascending aortic aneurysms (11–17).
We hypothesized that transient reduction of left ventricular function after the Maze procedure is related to denervation and can be assessed using 123I-MIBG. In the present study, using 123I-MIBG, we evaluated the serial changes in cardiac sympathetic nerve distribution and function after the Maze procedure.
MATERIALS AND METHODS
Patients
Thirteen patients with MV disease were enrolled in this study (Table 1). Six of them (5 men, 1 woman; mean age, 59.0 ± 9.2 y) had undergone MV repair or replacement and the Maze procedure (Maze+), and 7 (5 men, 2 women; mean age, 56.4 ± 17.8 y) had undergone MV repair or replacement without the Maze procedure (Maze−).
Preoperative Characteristics
One patient of the Maze+ group and 2 of the Maze− group took β-blockers preoperatively. The patients of the Maze+ group and 1 of the Maze− group continued to take it at 10 d and at 1 y after surgery, and 1 patient of the Maze− group stopped medication after surgery.
Performance of the Maze procedure was fundamentally the same as that initially described by Cox et al. (3). Both the left atrial appendage and the right atrial appendage were excised, the pulmonary veins were encircled completely, and atrial incisions were made to interrupt the conduction routes of the most reentrant circuits and direct the sinus impulse from the sinoatrial node to the atrioventricular node along a specified route.
Preoperative characteristics did not differ significantly between the Maze+ group and the Maze− group (Table 1). All 6 patients in the Maze+ group and 5 of the 7 patients in the Maze− group had AF, and 2 patients in the Maze− group had sinus rhythm.
None of the patients had significant coronary artery stenosis (luminal narrowing >50%, as shown by coronary angiography), diabetes mellitus, systemic autonomic disease, or cardiomyopathy. Patients with major complications after surgery were excluded.
Study Protocol
All patients underwent cardiac 123I-MIBG imaging preoperatively and 10 d and 1 y after surgery. Radionuclide ventriculography was performed 3 d after each 123I-MIBG imaging.
Cardiac 123I-MIBG Scintigraphy
123I-MIBG (111 MBq) (Dai-ichi Radioisotope Laboratory) was injected intravenously into the patients under resting and fasting conditions. Fifteen minutes and 4 h after injection, static planar images were acquired in the anterior view with a dual-head γ-camera (ADAC Vertex Plus; Phillips) equipped with low-energy, general-purpose collimators. Static images on 512 × 512 matrices were collected for 5 min with a 20% window centered at 159 keV. Subsequently, SPECT of the heart was performed in 64 × 64 matrices using a filtered backprojection method for reconstruction. A ramp filter and a Butterworth filter with an order of 5.0 and a cutoff frequency of 0.50 cycle per pixel were used for reconstruction. No attenuation or scatter correction was performed.
Left ventricular uptake was assessed by quantitative analysis performed by manually drawing the region of interest over the left ventricle in the anterior view. Rectangular regions of interest of 9 × 9 pixels were placed over the upper mediastinum. Counts per pixel were calculated from each region of interest located in the heart and mediastinum. The heart-to-mediastinum count ratios of early planar images (H/M) were computed to quantify the cardiac uptake of 123I-MIBG (18).
The washout rate (WR) was calculated using the following formula:
 where H = mean counts per pixel in the left ventricle and M = mean counts per pixel in the upper mediastinum.
where H = mean counts per pixel in the left ventricle and M = mean counts per pixel in the upper mediastinum.
Radionuclide Ventriculography
Red blood cells were labeled with 370 MBq of 99mTc-pertechnetate in vivo 10 min after intravenous administration of stannous pyrophosphate. Imaging was performed with a dual-head γ-camera (Millennium MG; Elgems) equipped with low-energy, high-resolution collimators. Data were acquired on 64 × 64 matrices using 16 frames per cardiac cycle with electrocardiographic gating during the equilibrium state. The images were transferred to a GENIE computing system (GE Yokogawa Medical System) for analysis, and the left ventricular ejection fraction (LVEF) was calculated automatically.
Statistical Analysis
All results are expressed as the mean ± SD. Continuous clinical data in subgroups of patients were compared using the Mann–Whitney test. Potential differences in sex and the existence of AF between the Maze+ group and the Maze− group were analyzed by the 1-sided χ2 contingency table method. The Wilcoxon signed rank test was used to compare scintigraphic results before and after surgery. P < 0.05 was considered statistically significant.
RESULTS
Preoperative Characteristics
The preoperative LVEFs did not differ between the Maze+ group and the Maze− group (60.3 ± 6.9 vs. 53.0 ± 12.3, respectively; P = not significant [NS]). The H/M and WR were not significantly different between the 2 groups (H/M: 1.90 ± 0.25 vs. 1.94 ± 0.32, respectively; P = NS; WR: 39.9 ± 6.5 vs. 40.4 ± 5.1, respectively; P = NS).
Serial Changes in LVEF
The LVEF of the Maze+ group significantly decreased 10 d after surgery (44.2 ± 4.8) compared with that before surgery (60.3 ± 6.9; P < 0.05) and significantly increased at 1 y (65.2 ± 2.9) compared with that at 10 d (P < 0.05). In the Maze− group, there was no significant change in the LVEF at 10 d (53.0 ± 12.3) or 1 y (58.6 ± 4.8) after surgery compared with that before surgery (60.4 ± 4.6) (Fig. 1).
Serial changes of LVEFs in both groups: Maze+ (A) and Maze− (B). Pre = preoperative LVEF; 10 d = LVEF 10 d after surgery; 1 y = LVEF 1 y after surgery.
Changes in 123I-MIBG Images in Early Stage After Surgery
Regular sinus rhythm was restored in all patients of the Maze+ group 10 d after surgery. In the Maze− group, the 2 patients who were in sinus rhythm before surgery remained in sinus rhythm after surgery and the 5 other patients remained in AF.
In the Maze+ group, the H/M was significantly lower 10 d after surgery (1.51 ± 0.18) than that at the preoperative stage (1.90 ± 0.25; P < 0.05). In the Maze− group, the H/M at 10 d (2.06 ± 0.18) did not differ from that at the preoperative stage (1.94 ± 0.32; P = NS) (Fig. 2).
Serial changes in H/Ms of both groups: Maze+ (A) and Maze− (B). Pre = preoperative H/M; 10 d = H/M 10 d after surgery; 1 y = H/M 1 y after surgery.
The WR 10 d after surgery (46.9% ± 3.4%) was significantly higher in the Maze+ group than that at the preoperative stage (39.9% ± 6.5%; P < 0.05) and that in the Maze− group (37.0% ± 5.1%) was significantly lower than that before surgery (40.4% ± 5.1%; P < 0.05) (Fig. 3).
Serial changes in WRs of both groups: Maze+ (A) and Maze− (B). Pre = preoperative WR; 10 d = WR 10 d after surgery; 1 y = WR 1 y after surgery.
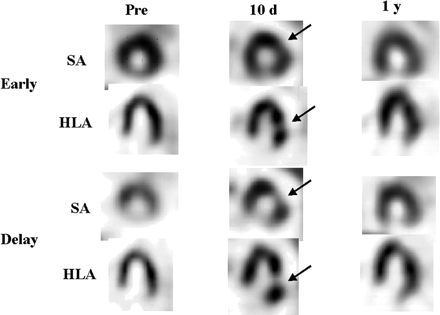
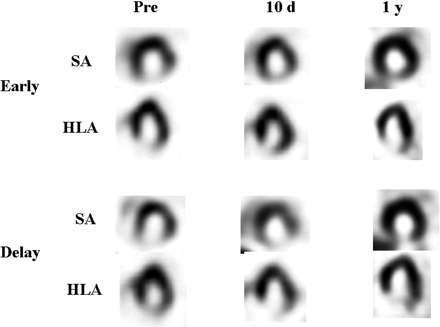
On the regional observation, SPECT images of the patients in the Maze+ group showed a decrease in the retention of 123I-MIBG in the lateral wall in all 6 patients, whereas none of the patients in the Maze− group exhibited regional changes (Figs. 4 and 5).
A 68-y-old man (patient 2) who had undergone MV replacement with Maze procedure. Reduction in uptake of 123I-MIBG and increase in WR were observed 10 d after surgery in lateral region (arrows). These changes resolved 1 y after surgery. Pre = preoperative images; 10 d = images 10 d after surgery; 1 y = images 1 y after surgery; SA = short-axis images; HLA = horizontal long-axis images.
A 60-y-old woman (patient 12) who had undergone MV replacement without Maze procedure. Distribution of 123I-MIBG exhibited no regional change after surgery. Pre = preoperative images; 10 d = images 10 d after surgery; 1 y = images 1 y after surgery; SA = short-axis images; HLA = horizontal long-axis images.
Changes in 123I-MIBG Images in Late Stage After Surgery
Regular sinus rhythm was restored in all patients of the Maze+ group 1 y after surgery. In the Maze− group, the 2 patients who were in sinus rhythm before surgery remained in sinus rhythm after surgery and the 5 other patients remained in AF.
The H/M in the Maze+ group 1 y after surgery (2.23 ± 0.18) was significantly higher than that 10 d after surgery (1.15 ± 0.18; P < 0.05) (Fig. 2). In the Maze− group, the H/M 1 y after surgery (2.13 ± 0.17) tended to be higher than that 10 d after surgery (2.06 ± 0.18) but was not significantly higher (P = NS) (Fig. 2).
The WR 1 y after surgery was significantly lower in both groups (Maze+, 36.7% ± 6.1%; Maze−, 32.9% ± 2.5%) than that 10 d after surgery (Maze+, 46.9% ± 3.4%, P = 0.028; Maze−, 37.0% ± 5.1%, P < 0.05) (Fig. 3).
On SPECT images, the decrease in the retention of 123I-MIBG in the lateral wall improved 1 y after surgery in all 6 patients of the Maze+ group (Fig. 4).
Correlation Between H/M, WR, and LVEF
Changes in the H/M in the early and late stages after surgery for both groups showed a significant correlation with changes in the LVEF (r = 0.82; P < 0.05), and changes in the WR showed a significant inverse correlation with changes in the LVEF during these stages (r = −0.81; P < 0.05) (Fig. 6). Changes in these factors were calculated using the value 10 d after surgery minus the preoperative value or the value 1 y after surgery minus the value 10 d after surgery.
Correlation between changes in H/M, WR, and LVEF: correlation between changes in H/M and LVEF (A) and that between changes in WR and LVEF (B). •, Changes between before surgery and 10 d after surgery in Maze+ group; ○, those of Maze− group; ▪, changes between 10 d and 1 y after surgery in Maze+ group; □, those of Maze− group.
DISCUSSION
To our knowledge, this study has demonstrated for the first time a transient decrease in 123I-MIBG uptake with increased washout in the early stage, with recovery in the late stage, after the Maze procedure. In patients with MV disease, such serial adrenergic neuronal changes may be related with changes in the LVEF after surgery.
MIBG is an analog of the adrenergic false neurotransmitter guanidine and is taken up by myocardial sympathetic postganglionic presynaptic neurons by an energy-dependent mechanism in a manner similar to norepinephrine (10,18). It is trapped in vesicles but is not catabolized by either monoamine oxidase or cathechol O-methyltransferase (19).
The initial uptake of 123I-MIBG reflects the number and function of norepinephrine transporters (18). In this study, the H/M after the Maze procedure decreased in the early stage and improved in the late stage after surgery. This result indicates denervation and reinnervation after the Maze procedure combined with MV repair or replacement. In the patients who had undergone MV repair or replacement without the Maze procedure, the H/M showed no significant change in both the early stage and the late stage, indicating that these changes in the H/M after this procedure were not influenced by MV surgery itself.
The washout of 123I-MIBG can be used as a global marker of sympathetic nerve activity (18). An increase in washout has been observed in heart failure caused by various diseases (20–22). In this study, the WR increased in the early stage and normalized in the late stage after the Maze procedure, suggesting transient changes in global sympathetic nerve function. These changes may reflect serial changes in the LVEF after this procedure.
The reduction in the LVEF in the early stage after the Maze procedure can be explained by several factors, such as the disappearance of atrial contraction, the longer duration time of cardiopulmonary bypass, aortic cross-clamping, and cardiac arrest among others (1,7). In our study, changes in the LVEF had a significant correlation with those in 123I-MIBG myocardial uptake and the WR. This phenomenon suggests that the function of sympathetic nerve activity may influence, at least in part, the change in the LVEF after the Maze procedure.
On the regional observation, the decrease in 123I-MIBG uptake was most severe in the lateral regions. The left lateral cardiac nerve, which is one of the sympathetic nerves, originates from the dorsal plexus, passes posterior to the left atrial appendage and anterior to the left pulmonary veins, and distributes to the lateral wall of the left ventricle (23,24). The incision of the left atrial wall associated with the Maze procedure may injure the left lateral cardiac nerve and induce the denervation of the left ventricle. In the late stage after the Maze procedure, the H/M value was restored. This change seems to be quite similar to the scintigraphic uptake of a norepinephrine analog after heart transplantation, the arterial switch operation, and surgical repair of ascending aortic aneurysms (11–17). This result indicates reinnervation after the Maze procedure.
We believe that the observed alterations of myocardial 123I-MIBG uptake were mainly global in sympathetic neuronal function based of the significant changes in global 123I-MIBG parameters, such as the H/M and WR, rather than the regional change in the lateral wall. These global changes may affect the LVEF after the Maze procedure. Future studies are necessary to clarify whether the changes in global parameters were not solely the result of regional change.
Tamai et al. indicated that the sinoatrial node response to exercise was attenuated early after the Maze procedure and improved at the late stage after surgery (9). The sinoatrial node is controlled not by the left lateral cardiac nerve but by the sympathetic nerve of the right coronary cardiac nerve, and the spatial resolution of SPECT scanners does not currently allow the visualization of the atrial wall or sinus node. However, denervation and reinnervation may occur simultaneously in both the left lateral cardiac nerve and the right coronary cardiac nerve. Although we did not measure the autonomic function, such as the sinoatrial node response, our findings suggest that changes in the 123I-MIBG accumulation pattern of the left myocardium may reflect the serial changes in the sinoatrial node response. Further investigations are needed to determine this relationship.
The uptake of 123I-MIBG is affected by β-blockers. In this study, 1 patient of the Maze+ group and 2 of the Maze− group took β-blockers preoperatively, and 1 patient of the Maze− group stopped medication after surgery. Since there were no changes in 123I-MIBG findings or the LVEF in the patients of the Maze− group, we consider that β-blocker treatment had no, or only minor, influence in this study.
Recovery of AF may possibly alter sympathetic nerve function in relation to changes in left ventricular function. However, AF remained in recovery all of the time after the Maze procedure, whereas a transient decrease in sympathetic nerve function and left ventricular function occurred in the early stage with recovery in the late stage. Thus, a direct relation between the recovery of AF and the recovery of sympathetic nerve function and left ventricular function seems to be less likely.
Several study limitations should be addressed. First, we did not measure the norepinephrine levels of each patient. A high plasma norepinephrine level has been reported to inhibit 123I-MIBG accumulation and accelerate washout of the heart (19). There is a possibility that the decrease in the H/M and the increase in the WR after the Maze procedure may be affected by the plasma norepinephrine level. However, the sympathetic nerve function did not change in the early stage in the Maze− group. In addition, accumulation of 123I-MIBG decreased, particularly in the lateral region not in the entire region, on the SPECT images. Therefore, these changes after the Maze procedure are not likely to be caused by the plasma norepinephrine level.
Also, we did not measure the autonomic function, such as sinoatrial node response, chronotropic function, and so on. However, we acquired our patients’ images at the same periods as earlier studies, which reported the temporary attenuation in sinoatrial function and the LVEF. Further investigations are necessary to determine the relation between 123I-MIBG accumulation and autonomic function.
Another limitation is the limited size of the patient population. However, the current study, with a limited number of patients, clearly showed a significant correlation between changes in sympathetic nerve function and those of left ventricular function after the Maze procedure. Further studies are warranted to confirm our preliminary results and also to determine the prognostic value of the recovery of the sympathetic function in the late stage after the Maze procedure.
CONCLUSION
Cardiac sympathetic nerves were denervated early after and reinnervated late after the Maze procedure. Such autonomic function changes can be serially assessed using 123I-MIBG scintigraphy. The adrenergic nerve changes may be correlated, at least in part, with the transient decrease in left ventricular function after this procedure.
Footnotes
Received July 11, 2004; revision accepted Mar. 23, 2005.
For correspondence or reprints contact: Nagara Tamaki, MD, Department of Nuclear Medicine, Hokkaido University Graduate School of Medicine, N-15, W-7, Kita-ku, Sapporo 060-8638, Japan.
E-mail: natamaki{at}med.hokudai.ac.jp