Abstract
The aim of this study was to evaluate the neuroprotective effect of TRA-418, an antiplatelet agent, using PET in a monkey model of stroke. TRA-418 is a nonprostanoid compound with dual action: antagonistic effects on thromboxane A2 receptors and agonistic effects on prostaglandin I2 receptors. Methods: Via a transorbital approach, cynomolgus monkeys underwent a 3-h occlusion of the right middle cerebral artery (MCA), followed by reperfusion and observation for 4 d. Starting 2 h after the MCA occlusion, TRA-418 was administered at low and high doses (6 animals at each dose). Six control animals received a bolus and infusion of drug vehicle after MCA occlusion. Steady-state 15O continuous inhalation was used for assessment of local cerebral blood flow, cerebral metabolic rate of oxygen, and oxygen extraction fraction using high-resolution PET. Five consecutive PET scans (before occlusion; 2 h after occlusion; and 2 h, 24 h, and 4 d after reperfusion) were obtained for each monkey. The extent of the cerebral damage due to ischemia was measured histologically at 4 d after reperfusion. Results: Histologic observation 4 d after MCA occlusion showed that cerebral damage was less (P = 0.05) in animals treated with high-dose TRA-418 than in control animals. Although not affecting cerebral blood flow during the experiments, treatment with TRA-418 significantly (P < 0.05) suppressed reduction of the cerebral metabolic rate of oxygen after reperfusion. Conclusion: Our observations suggest that TRA-418 has neuroprotective action, as displayed in a primate model of stroke using PET monitoring.
Platelet activation occurs in acute cerebral infarction, and clinical trials of antiplatelet therapy have revealed the advantages of acetylsalicylic acid use in this situation (1,2). Recently, an antiplatelet agent, glycoprotein IIb/IIIa receptor antibody (ReoPro; Eli Lilly), that strongly inhibits induced platelet aggregation, has been developed for treatment of acute stroke (3). However, the effect profile of this product includes a tendency toward severe bleeding, and its use may increase the risk of intracerebral hemorrhage (4). TRA-418 is a new nonprostanoid compound (Toray Industries, Inc.) with dual action: antagonistic action on thromboxane A2 receptors and agonistic action on prostacyclin receptors (5,6). Although TRA-418 strongly inhibits platelet aggregation, this agent has not been found to lead to increased bleeding in animal studies (Toray Industries, Inc., unpublished data, 2000). Because of its weak vasodilating effect, TRA-418 can inhibit platelet aggregation without a decrease in systemic blood pressure (6). These findings suggest that TRA-418 may be a therapeutic candidate for treatment of acute stroke.
Recent clinical studies on acute stroke have revealed a discrepancy between the preclinical and clinical effectiveness of neuroprotective agents (7). This discrepancy may be due to differences between the reactions of humans experiencing stroke and of animal species used in experimental stroke models. Almost all published preclinical experiments have been performed using rodent stroke models rather than primate models, whose outcome may more closely resemble the effects we would see in humans treated for stroke. Therefore, it is important to evaluate the effects of agents in a suitable nonhuman primate model of stroke before clinical trials are initiated.
PET has proven to be a powerful tool with which to investigate the pathophysiology of stroke (8,9). We have demonstrated that PET studies in monkey models of stroke were useful for characterization of the acute phase of cerebral ischemia and for investigation of the effects of neuroprotective agents (8,9). In the present study, we used PET to investigate the neuroprotective effect of TRA-418 in a monkey model of stroke.
MATERIALS AND METHODS
Animal Preparation
The studies were performed on 18 male cynomolgus monkeys (Macaca fascicularis; Clea Japan, Inc.) with body weights ranging from 4.29 to 6.61 kg. All experiments were performed in accordance with the institutional guidelines of the Central Research Laboratory of Hamamatsu Photonics.
The procedure for animal preparation used the technique of Takamatsu et al. (8,9). Briefly, a 10-mg dose of intramuscular ketamine hydrochloride per kilogram of body weight was used to induce anesthesia. The monkeys were nontracheotomized, artificially ventilated, and immobilized with 0.05 mg of intramuscular pancuronium bromide per kilogram of body weight every 2 h. Anesthesia was maintained with isoflurane (concentration, 0.6%–0.8%) in a 7:3 N2O:O2 gas mixture during the entire experiment. The left femoral artery was catheterized to enable measurement of mean arterial blood pressure and heart rate and sampling of arterial blood. Mean arterial blood pressure, heart rate, and rectal temperature were continuously monitored, and arterial partial pressures of oxygen and carbon dioxide, pH, and plasma glucose levels were periodically monitored.
Occlusion Protocol
The right middle cerebral artery (MCA) was occluded using a transorbital approach (8,9) as described below. After administration of 0.05 mg of intramuscular atropine per kilogram of body weight, the right globe was removed. The head of the monkey was fixed in an acrylic stereotactic apparatus (SFCT-RB-PR-2; Hamamatsu Photonics) that had already been fixed in the PET scanner gantry (SHR7700; Hamamatsu Photonics) using a laser beam localization system to allow for reproducible head positioning. After a 30-min 68Ga–68Ge transmission scan and a baseline PET scan had been obtained, the monkey was moved to an operating table and the procedure performed under an operating microscope. A craniotomy was performed superolateral to the optic nerve. After the dura had been opened, the MCA was occluded with 2 microvascular clips (Sugita Aneurysm Mini Clip; Mizuho Ikakogyo Co. Ltd.), one on the proximal part of the main MCA trunk and the other on the distal-to-orbitofrontal branch of the vessel. Upon completion of the occlusion procedure, the monkey was fixed into the stereotactic apparatus; after a 30-min 68Ga–68Ge transmission scan had been obtained, additional PET scans (postischemic scans) were obtained. During the experiments, body temperature was maintained within normal limits with heated blankets, and the physical parameters of the animal were monitored. The microvascular clips were removed 3 h after placement, without removing the animal from the stereotactic apparatus.
PET Studies
Five consecutive PET scans were obtained for each monkey (Fig. 1) using an SHR7700 system (10). The first scan was obtained before MCA occlusion (preischemic baseline scan). The second scan was obtained about 2 h after the start of occlusion (1 h before the start of reperfusion). The third and fourth scans were obtained about 2 and 24 h, respectively, after reperfusion. The final scan was obtained 4 d after occlusion. Cerebral blood flow (CBF), cerebral metabolic rate of oxygen (CMRO2), oxygen extraction fraction (OEF), and local cerebral blood volume were assessed using the steady-state 15O inhalation method (11,12), with successive inhalation of trace amounts of C15O2 (0.8 GBq/min), 15O2 (2 GBq/min), and C15O (4 GBq/min) (11,12). Each 15O gas was administered through a ventilator (15 strokes/min, 70 mL/stroke). The C15O2 and 15O2 scans were started after the PET camera gantry reached radioisotope saturation; the C15O scan was started 2 min after a 20-s inhalation of C15O gas. Each scan was acquired over 5 min and consisted of five 1-min frames. During each scan, 2 arterial blood samples were taken (one at the beginning and one at the end of data acquisition) to monitor whole-blood and plasma radioactivity. The mean values for whole-blood and plasma radioactivity were used for parametric image generation (13). The calculated CMRO2 and OEF values were obtained after correction for cerebral blood volume.
The experimental procedure.
The wound was closed after the completion of the third PET scan, 2 h after the reperfusion, and anesthesia was then stopped. The animals were treated with painkillers. At the fourth PET scan, 24 h after the reperfusion, the animals were anesthetized again, and anesthesia was then stopped after the PET scan. At the last PET scan, 4 d after the reperfusion, the animals were anesthetized again. After the completion of the PET scan, the heart was stopped by intravenous administration of a high dose of pentobarbital.
Determination of CBF, OEF, and CMRO2 Changes
The procedure for calculation of each PET parameter was slightly modified from that of Takamatsu et al. (8,9). Each CBF, OEF, or CMRO2 value obtained from the contralateral intact cerebral side was assumed to be unchanged throughout scanning, and each value obtained from the second to fifth scans was normalized to that of the first scan of the normal control condition by use of an image analysis system (Alice; Perceptive Informatics). Each normalized PET image was analyzed using a slightly modified procedure described by Takamatsu et al. (9).
Drug Administration
TRA-418 was administered starting 2 h after MCA occlusion as either a 5 μg/kg/min bolus injection for 0.5 h followed by a 2.5 μg/kg/min continuous infusion for 23.5 h (low-dose TRA-418; n = 6) or as a 15 μg/kg/min bolus injection for 0.5 h followed by a 7.5 μg/kg/min continuous infusion for 23.5 h (high-dose TRA-418; n = 6) (Fig. 1). Doses of TRA-418 were selected on the basis of preclinical data showing 20% or 90% inhibition (low-dose and high-dose TRA-418, respectively) of adenosine diphosphate–induced platelet aggregation (12). Six control animals received a bolus and infusion of drug vehicle after MCA occlusion in the same manner as for the drug.
Neuropathology
Four days after MCA occlusion, the monkeys were deeply anesthetized (and eventually euthanized) with sodium pentobarbital. After saline perfusion at 100 mm Hg, the brain was fixed via transcardial perfusion using a 10% formalin neutral buffer solution, pH 7.4. The brain was then removed and embedded in paraffin. Sections 10 μm thick were cut and were stained with hematoxylin and eosin. In 2 coronal slices (18 or 24 mm anterior to interaural), the neuronal damage was defined (8) and the area of neuronal damage measured using a computerized image analysis system (Photoshop; Adobe). The volumes of neuronal damage were calculated from the areas of damage in each coronal section and the respective anteroposterior coordinates.
Data Analysis
Data are presented as the mean ± SE. All data were evaluated using ANOVA, followed by the Fisher test. A P value of <0.05 was considered significant.
RESULTS
Physiologic variables were measured during anesthesia and remained within the reference range (Tables 1 and 2).
Physiologic Parameters for Glucose and Partial Pressures of CO2 and O2
Physiologic Parameters for Mean Arterial Blood Pressure, Heart Rate, and Rectal Temperature
Neuropathologic Study
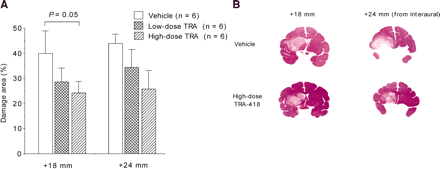
Figure 2A shows the effect of TRA-418 on the extent of cerebral damage 4 d after MCA reperfusion. MCA occlusion and reperfusion caused cerebral damage in both the cortex and the basal ganglia, as seen 4 d after the reperfusion (Fig. 2B). TRA-418 at a high dose tended to reduce the extent of cerebral damage (P = 0.05). In this study, no side effects such as intracerebral bleeding were observed.
(A) Effect of TRA-418 on extent of cerebral damage. TRA-418 at high dose reduced damaged area in coronal slices (18 mm anterior to interaural) (P = 0.05). Data are presented as mean ± SE (n = 6). (B) Photographs of typical coronal slices (18 and 24 mm anterior to interaural) in animals treated with vehicle or high-dose TRA-418.
PET Study
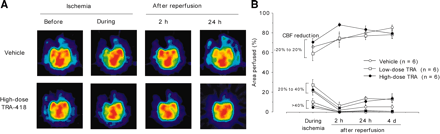
Figure 3A shows typical PET images of CBF in animals treated with vehicle or TRA-418 at the high dose. In the animals treated with vehicle, MCA occlusion reduced CBF in the cortex and basal ganglia. CBF levels increased at 2 h after reperfusion but did not return to preischemic values. TRA-418 did not affect the CBF changes caused by MCA occlusion and reperfusion (Fig. 3B).
(A) PET images of typical CBF before and during ischemia and at 2 and 24 h after reperfusion in animals treated with vehicle or high-dose TRA-418. (B) Effect of TRA-418 on CBF. No significant differences were found between animals treated with vehicle and animals treated with either TRA-418 dose. CBF reduction was calculated by dividing each PET image of cerebrum by its baseline image and determining the areas of −20% to 20%, 21% to 40%, and >40% change in CBF. Areas were calculated as percentage area divided by total hemispheric area of nonischemic side.
Figure 4A shows typical CMRO2 PET images at 24 h and 4 d after reperfusion in animals treated with vehicle or high-dose TRA-418. Figure 4B shows the volume change of CMRO2 levels, which decreased by more than 40% compared with CMRO2 values in the first scan of control animals after MCA occlusion and reperfusion. In the vehicle control group, areas of low CMRO2 increased during ischemia and slightly recovered when reperfused; however, at 24 h after reperfusion, the area of CMRO2 reduction was similar to that seen during induced ischemia. High-dose TRA-418 significantly suppressed CMRO2 reduction after reperfusion (P < 0.05).
(A) PET images of typical CMRO2 at 24 h and 4 d after reperfusion in animals treated with vehicle or high-dose TRA-418. (B) Effect of TRA-418 on CMRO2 reduction, where CMRO2 decreased by more than 40% compared with preischemic scan. High-dose TRA-418 significantly suppressed (P < 0.05) CMRO2 reduction at 24 h after reperfusion. Data are presented as mean ± SE. Areas were calculated as percentage area divided by total hemispheric area of nonischemic side.
Figure 5 shows the change in OEF after MCA occlusion and reperfusion. OEF decreased by more than 40%, compared with the values in the first scan of normal control animals. In the vehicle control group, the area of OEF reduction increased remarkably after reperfusion was started. High-dose TRA-418 significantly inhibited the OEF reduction caused by reperfusion (P < 0.05).
Effect of TRA-418 on OEF reduction, where OEF decreased by more than 40% compared with preischemic scan. High-dose TRA-418 significantly (P < 0.05) inhibited OEF reduction. Data are presented as mean ± SE.
Figure 6 shows a good correlation (P = 0.0015) between the damaged area observed histologically and the areas of more than 40% CMRO2 reduction.
Correlation between >40% decrease in CMRO2, compared with preischemic scan, and amount of brain damage (histologically confirmed) at 4 d after reperfusion.
DISCUSSION
In the present study, we demonstrated that TRA-418 is effective in a nonhuman primate model of stroke using PET monitoring. TRA-418 histologically reduced cerebral damage due to ischemia and subsequent reperfusion. Although not improving CBF after ischemia and reperfusion, TRA-418 was effective in suppressing CMRO2 and OEF reduction after reperfusion.
Plasma thromboxane A2 levels have been reported to increase in patients with acute stroke (14). Thromboxane A2 causes platelet aggregation and vasoconstriction, and reactive oxygen is produced via thromboxane A2 synthesis. These factors may contribute to the progression of cerebral damage (14). Acetylsalicylic acid suppresses thromboxane A2 generation by inhibition of cyclooxygenase and has been reported in clinical trials to be effective in treating acute stroke (15,16). However, acetylsalicylic acid also inhibits prostaglandin I2 production in the endothelium, and prostaglandin I2 inhibits platelet aggregation and acts as a vasodilating agent. Prostaglandin I2 analogs have been reported to prevent ischemic neuronal damage in gerbils and rats, probably because of vasodilating action and inhibition of platelet aggregation (17,18). The suppression of prostaglandin I2 production is a disadvantage for the treatment of acute stroke. TRA-418 is a nonprostanoid compound with dual action: an antagonistic action on thromboxane A2 receptor and an agonistic action on prostaglandin I2 receptor. These findings suggest that TRA-418 may be a valid candidate for the treatment of acute stroke.
The present study found a good correlation between the damaged area of brain observed histologically and the area of CMRO2 reduction measured using PET at 4 d after ischemia and reperfusion. We previously demonstrated a similar correlation between histologically damaged areas and areas of CMRO2 reduction at 8 h after ischemia and reperfusion (8,9). These findings suggest that the area of CMRO2 reduction (measured by PET) may predict the extent of cerebral damage after an ischemic event. In the present study, TRA-418 seemed to suppress CMRO2 reduction after reperfusion, suggesting that TRA-418 may protect against reperfusion injury. The effect of TRA-418 is similar to that of FK506 reported by Takamatsu et al. using the same model (9). Further, a study has shown that acute crossed cerebellar diaschisis and its serial changes on PET images after reperfusion in acute stroke patients can predict the volume of supratentorial hypoperfusion and clinical outcome (19). From these findings, using PET images, one should expect to predict prognosis in acute stroke.
We previously demonstrated that reduction of the OEF might be a useful indicator of reperfusion injury (8,9). OEF increase during ischemia reflects the inadequate supply of oxygen for underlying metabolic needs. Hyperperfusion after reperfusion provides an excess of oxygen that may be adequate for production of reactive oxygen radicals, leading to accelerated brain damage. In the present study, TRA-418 suppressed the OEF reduction caused by reperfusion, also suggesting that TRA-418 may protect against reperfusion injury.
In this study, the observation period was up to 4 d after the MCA occlusion. It has been reported that in clinical situations, observation is required for up to 3 mo after the onset of stroke. However, in this study, PET parameters did not differ between 24 h and 4 d after reperfusion. Accordingly, the effects of TRA-418 could be evaluated in this study as reported. In this study, we analyzed the effects of TRA-418 using a normalized PET image as reported by Takamatsu et al. (9). When changes in PET parameters are observed over time, PET scanning is performed several times, increasing the sampling blood volume for calculating the absolute values affecting CBF and blood pressure. Therefore, we decided to analyze the effects of TRA-418 using normalized PET images. In this study, we used 2 coronal sections to evaluate the effects of TRA-418 in an area of ischemic injury. Based on our previous reports (8,9) and that of Kito et al. (20), the 2 sections that were used in this study indicate the largest area of infarction due to MCA occlusion in monkeys. Thus, these 2 sections could be used to evaluate the effects of TRA-418.
In the present study, we demonstrated that TRA-418 was effective in treating the development of cerebral infarction in a monkey model of stroke with PET monitoring. The results of this study indicate that TRA-418 may inhibit reperfusion injury. Further, our data suggest that continuous PET monitoring may predict the time course of cerebral infarct development and can help us to determine when neuroprotective agents may be most useful in preventing the development of cerebral infarction.
Acknowledgments
This work was supported by CREST, Japan Science and Technology Agency; and by Toray Industries, Inc. Naohiro Yamada and Hirotoshi Matsuura are employees of Toray Industries, Inc., which is the manufacturer of TRA-418. Naohiro Yamada is one of the inventors of TRA-418 and is named in the patents of TRA-418–related compounds. Both Naohiro Yamada and Hirotoshi Matsuura will receive an incentive from Toray Industries, Inc., after commercialization of TRA-418.
Footnotes
Received Apr. 13, 2005; revision accepted Aug. 3, 2005.
For correspondence or reprints contact: Kazuo Umemura, MD, PhD, Department of Pharmacology, Hamamatsu University School of Medicine, 1-20-1 Handayama, Hamamatsu, 431-3192, Japan.
E-mail: umemura{at}hama-med.ac.jp