Abstract
Although autonomic function has been investigated in panic disorder, previous studies have not yet revealed a consistent autonomic change in this disease. The purpose of this study was to evaluate the cardiac sympathetic function in panic disorder using 123I-metaiodobenzylguanidine (123I-MIBG). Methods: Myocardial imaging using 123I-MIBG was performed on 9 patients with panic disorder (7 men, 2 women; mean age, 37.4 ± 13.2 y) and 11 control subjects (11 men; mean age, 37.6 ± 9.3 y). Early (30 min) and delayed (4 h) planar images were taken after the injection of 111 MBq 123I-MIBG. The mean counts in the whole heart and the mediastinum were obtained from the early and delayed images to calculate the heart-to-mediastinum count ratios (H/M ratios) and the myocardial washout rate. Results: The 123I-MIBG H/M ratios of the patients with panic disorder were 1.80 ± 0.16 for the early images and 1.86 ± 0.30 for the delayed images, which were significantly lower than those of the control subjects (2.15 ± 0.15 [P = 0.001] and 2.26 ± 0.21 [P = 0.009], respectively). The 123I-MIBG washout rate from the heart in the patients with panic disorder (33.8% ± 6.9%) was significantly higher than that in the control subjects (27.8% ± 3.5%) (P = 0.02). Conclusion: 123I-MIBG myocardial scintigraphy demonstrated impairment of cardiac sympathetic function in panic disorder. The results suggest that 123I-MIBG imaging could become a useful tool for analyzing the pathophysiology of panic disorder.
Patients with panic disorder experience recurrent panic attacks, which are characterized by episodes of intense anxiety accompanied by a range of alarming somatic symptoms, such as palpitations, tachycardia, sweating, shaking, shortness of breath, and chest pains.
These findings are suggestive of autonomic nervous system involvement in panic disorder. However, previous studies on autonomic nervous system function in panic disorder have yielded conflicting results.
Metaiodobenzylguanidine (MIBG) is a physiologic analog of norepinephrine and is actively transported into the norepinephrine granules of sympathetic nerve terminals by the norepinephrine transporter (1–3). 123I-MIBG myocardial scintigraphy has been used for the evaluation of cardiac sympathetic function in a variety of heart diseases, including heart failure, coronary artery disease, and cardiomyopathy (4–8). Recently, the usefulness of 123I-MIBG myocardial imaging has also been documented in patients with autonomic failure associated with various neurologic diseases of the central and peripheral nervous system, such as Parkinson’s disease, multiple system atrophy, and dementia with Lewy bodies (9–11). In the present study, we tested cardiac sympathetic nervous function in patients with panic disorder using 123I-MIBG.
MATERIALS AND METHODS
Subjects
The study was performed on 9 patients with panic disorder (7 men, 2 women; age range, 16–60 y; mean age, 37.4 ± 13.2 y) and 11 age-matched healthy control subjects (11 men; age range, 26–52 y; mean age, 37.6 ± 9.3 y). None of the patients experienced panic or near-panic experiences in the 123I-MIBG study. The patients were diagnosed as having panic disorder according to the DSM-IV criteria at the neuropsychiatric outpatient clinic of our institution (12). The patient profiles are summarized in Table 1. The mean duration of illness for the patients was 5.3 y (range, 3.2–8.4 y). The disease severity was from stage 2 to stage 7 (mean, stage 4.4) according to Sheehan’s grading of the clinical course of panic disorder (13). None of the patients had diabetes mellitus or heart diseases, including ischemic heart disease, cardiomyopathy, hypertensive heart disease, and congestive heart disease. Two patients were drug free, and the remaining 7 patients had been treated with alprazolam, which was discontinued at least 3 d before the 123I-MIBG examination. None of the patients was receiving drugs that may have interfered with 123I-MIBG uptake by sympathetic nerve terminals, such as tricyclic antidepressant drugs, reserpine, and clonidine. Informed consent was obtained from all patients and control subjects before enrollment.
Patient Characteristics
123I-MIBG Studies
Patients, at rest, were injected with 111 MBq 123I-MIBG (Daiichi Radioisotope Laboratories Co.) through an intravenous line that was established earlier. Thirty minutes and 4 h after the tracer administration, static planar images of the chest in the anterior view were acquired for 4 min in a 256 × 256 matrix using a γ-camera with a large field of view and a low-energy, high-resolution collimator (E.CAM; Siemens Medical Systems).
The planar 123I-MIBG images were analyzed by a region-of-interest (ROI) technique to obtain semiquantitative parameters for tracer distribution (8). To assess the global myocardial kinetics of 123I-MIBG, an ROI was drawn manually over the whole heart. A second, rectangular ROI over the upper mediastinum was used as a reference background region. The 123I-MIBG count densities of the heart (H) and the mediastinum (M) were calculated from the early and delayed images. The heart-to-mediastinum count ratios (H/M ratios) were calculated for the early and delayed images. In addition, the myocardial washout rate (WR) of 123I-MIBG from the heart for 4 h was calculated according to the formula: WR (%) = 100 × (He − Hd)/He, where He is the early cardiac count density and Hd is the decay-corrected delayed cardiac count density.
Statistical Analysis
Data are expressed as the mean ± SD. The Mann–Whitney U test was used to compare the scintigraphically obtained parameters in the patients with those in the healthy control subjects. P < 0.05 was considered significant.
RESULTS
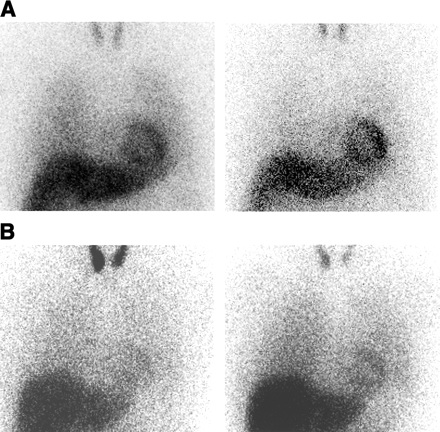
The scintigraphic results for the patients with panic disorder and the healthy control subjects are shown in Table 2. The 123I-MIBG H/M ratios of the patients with panic disorder were 1.80 ± 0.16 for the early images and 1.86 ± 0.30 for the delayed images, which were significantly lower than those in the control subjects (2.15 ± 0.15 [P = 0.001] and 2.26 ± 0.21 [P = 0.009], respectively). The 123I-MIBG WR from the heart in the patients with panic disorder (33.8% ± 6.9%) was significantly higher than that in the control subjects (27.8% ± 3.5%; P = 0.02). Scintigraphic examples of a patient with panic disorder (patient 3) and a healthy control subject are represented in Figure 1.
Myocardial 123I-MIBG anterior planar images (30 min [early image, left] and 4 h [delayed image, right]) of 52-y-old healthy control subject, with early H/M ratio of 2.31, delayed H/M ratio of 2.51, and WR of 26.1% (A) and of 39-y-old man with panic disorder (patient 3), with early H/M ratio of 1.63, delayed H/M ratio of 1.61, and WR of 36.1% (B).
Comparison of Scintigraphic Parameters of 123I-MIBG
DISCUSSION
No definitive pathophysiologic mechanism for panic disorder has been found to date. On the basis of the cardinal features of the symptoms of panic attacks—including palpitations, tachycardia, sweating, shaking, shortness of breath, and chest pains—autonomic nervous system dysfunction has been suggested as a possible pathology of panic disorder, and a variety of studies have attempted to measure autonomic nervous system function in panic disorder. However, these studies have failed to show consistent differences in autonomic nervous system function between patients with panic disorder and healthy subjects.
Studies have focused on the heart rate, blood pressure, and measurements of plasma or urinary catecholamines or catecholamine metabolites from an early stage of the investigation into panic disorder. Nesse et al. reported that patients with panic disorder had elevated heart rate, and plasma epinephrine and norepinephrine levels at rest, indicating baseline hypersensitivity of the cardiovascular autonomic nervous system function in panic disorder (14). On the contrary, although the heart rate and blood pressure increase in panic attacks, there have been reports that they do not differ between patients with panic disorder and healthy subjects during control periods (15,16). Stein and Asmundson performed a variety of tests consisting of postural challenge, isometric exercise, cold pressor, and the Valsalva maneuver and found that patients with panic disorder did not differ from healthy control subjects in their heart rate and blood pressure (17). They also measured plasma norepinephrine and epinephrine levels and concluded that patients with panic disorder showed catecholaminergic responses similar to those of healthy control subjects. Some investigators have reported elevation the plasma norepinephrine level (14,18), and others have reported that patients with panic disorder show no difference in the plasma norepinephrine level from that of healthy subjects even during panic attacks or after autonomic stimuli (17,19,20). One of the main reasons for this inconsistency is that perhaps the plasma norepinephrine concentration in samples obtained from the peripheral vein has important limitations. For example, since antecubital venous concentrations of norepinephrine primarily reflect the activity in muscle and skin sympathetic nerves to the forearm, they are difficult to use as a global or cardiac index of sympathetic activity (21). Wilkinson et al. used infusion of radiolabeled epinephrine and norepinephrine with arterial catheterization and coronary sinus sampling and showed that cardiac epinephrine overspill is increased at rest and during panic attacks in panic disorder (21). The method, which can give selective cardiac functional measurement, is rather complicated and it is possible that anxiety about the equipment and invasive procedures influence the physiologic features of the patients.
Power spectral analysis of heart rate variability has recently been used in various clinical disorders. It consists of high-frequency, low-frequency, and very-low-frequency components. The experimental results have suggested that the high-frequency component is mediated by the cardiac parasympathetic tone, which depends on respiration, whereas the low-frequency component is mediated by both the cardiac sympathetic and the parasympathetic tones (22–24). Generally, this body of research has indicated reduced overall heart rate variability, diminished vagal tone, and relative sympathetic dominance in patients with panic disorder (25–28). However, there have been reports that sympathetic activity is depressed and the vagal tone is predominant in panic disorder by heart rate variability analysis (29,30). In addition, there is the argument that since heart rate variability depends on both the parasympathetic and the sympathetic systems, together with intact baroreflex loops, it is a rather indirect measure of sympathetic function (31). Collectively, various alterations of autonomic nervous system function in panic disorder have been reported. There is no consensual explanation in the literature for these discrepant findings, and the discrepancies may, in part, be due to differences in the anxiety of the subjects at the time of testing or differences in the disease severity or duration among patients included across studies.
In the present study, we used 123I-MIBG as a direct approach for the assessment of cardiac sympathetic nervous function. It was demonstrated that the H/M ratio, as an index of myocardial 123I-MIBG uptake, was lower and the WR, as an index of myocardial clearance, was higher in patients with panic disorder than those values in age-matched healthy control subjects. The mechanisms for the abnormal findings of 123I-MIBG myocardial imaging, decreased H/M ratio, and increased WR are still under investigation. These phenomena are thought to indicate a derangement or imbalance in cardiac sympathetic nerve supply to the myocardium and are a common feature of damaged myocardium due to various diseases, such as heart failure, coronary artery disease, cardiomyopathies, and neurologic diseases (3–8). With respect to panic disorder, if the dramatic elevations in heart rate, blood pressure, and other alarming somatic symptoms generally observed in panic attacks are related to sympathetic activation, the recurrent surges of sympathetic overactivity by panic attacks could lead to depletion or exhaustion of the sympathetic efferent system, which can be depicted as an abnormality in 123I-MIBG myocardial imaging. Although further investigation is required to validate this possibility, 123I-MIBG myocardial imaging, which can be performed easily and noninvasively, would serve to explore the pathophysiology of panic disorder.
In addition to possibly contributing to the analysis of altered autonomic nervous system function in panic disorder, 123I-MIBG myocardial imaging may yield important insights into cardiac comorbidity in panic disorder. There have been reports showing an association between panic disorder and various cardiac illnesses, such as cardiovascular diseases, cardiomyopathy, and supraventricular tachycardia (32–35). Since panic disorder and these cardiac diseases can occur together, it is important to examine whether abnormal findings in an 123I-MIBG study are relevant to the development of cardiac illnesses in further investigations. In addition, since the study group in the present investigation was small and consisted of relatively advanced stages of panic disorder (stage 2 to stage 7; mean, stage 4.4), further studies with larger populations and various subgroups of disease severity are needed to clarify a more detailed role for 123I-MIBG in investigating panic disorder.
CONCLUSION
Our data indicate that patients with panic disorder appear to have significant myocardial sympathetic nervous dysfunction, as was demonstrated by abnormal 123I-MIBG imaging findings. The results suggest that 123I-MIBG imaging could be a useful and noninvasive tool for analyzing the pathophysiology of panic disorder.
Footnotes
Received Oct. 19, 2003; revision accepted Jan. 21, 2004.
For correspondence or reprints contact: Yoshio Tanabe, MD, Department of Radiology, Tottori University Hospital, 36-1 Nishichou, Yonago 683-8504, Japan.
E-mail address: ytanabe-ttr{at}umin.ac.jp