TO THE EDITOR:
We read with interest the article by Subbarayan et al. concerning the use of annexin V to image apoptosis in vivo (1). A series of publications in 1994 and 1995 had initiated a broad interest in the use of annexin V as a tool to measure apoptosis in vitro and in vivo. Hofstra et al. (2) and Narula et al. (3) augmented this interest by demonstrating that 99mTc-annexin V scintigraphy reveals apoptosis. Subbarayan et al. described a simplified method to produce 99mTc-annexin V. One part of the procedure was the heating of annexin V at 90°C for 10 min. In 1985, we reported that heating annexin V (at that time we called it a vascular anticoagulant) destroyed its phospholipid-binding activity, which determines its ability to target apoptotic cells (4). In 1997, van den Eijnde et al., who were the first to demonstrate that annexin V can be used to visualize apoptosis in vivo, showed that heat-inactivated annexin V does not bind to apoptotic cells in vivo (5). And Dumont et al. showed that the annexin V mutant, which does not bind to phosphatidylserine, fails to bind to apoptotic cells in vivo (6). Subbarayan et al. have not reported on the phospholipid-binding activity of their 99mTc-annexin V preparation, and we have serious doubts that the 99mTc-annexin V produced using their protocol would retain the potency to bind to apoptotic cells in vivo.
REFERENCES
REPLY:
We appreciate the comments of Reutelingsperger et al. We are aware of their cited references, which show that heat inactivation of annexin V prevents the protein from binding to phosphatidylserine and thus also from binding to apoptotic cells. Knowing this, we agree that it was quite bold of us to have even attempted the radiolabeling of annexin V with a method that used a temperature of 90°C for 10 min, as described in our paper (1). Thin-layer and size-exclusion chromatography confirmed that we had prepared the radiolabeled annexin V complex. We did not, however, test its phospholipid-binding properties, which could be done, for example, using the method described by Verbeke et al. (2). Our approach was instead to test the apoptosis-detecting properties of radiolabeled annexin V in tumor-bearing mice that had undergone photodynamic therapy 1 h before the annexin V application. That tumor uptake was rapid and significant is evident from Figures 4 and 5 of our paper (1). If the assertion of Reutelingsperger at al. that we destroyed the phosphatidylserine-binding properties of annexin V is true, then what accounts for our successful imaging? Could we have produced a 99mTc-complex—other than the annexin V—that found its way into the tumor? We sterile-filtrated the annexin V before injection, and such a complex would thus have at least been smaller than 0.22 μm. We did not, however, see any precipitate. Or could it have been that the additionally added compounds during the labeling, such as succinic dihydrazide, nicotinic acid, tricine, and propylenediaminetetraacetic acid, protected the annexin V molecule from degradation? Further research would be necessary to answer these questions.
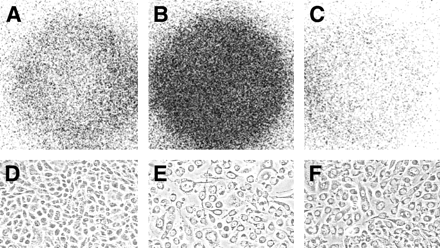
We have additional indications, obtained by a completely different set of experiments, that our method is able to produce a radiolabeled species that can detect apoptosis. In these, we used an in vitro cell culture system to grow melanoma cancer cells in 6-well plates. Both the right and the middle wells were incubated for 3 h with the photosensitizer Pc 4 (0.5 μmol/L), whereas the left well received no Pc 4. All wells were then subjected to photodynamic therapy. Three hours later, 4.8 MBq of the 99mTc-labeled annexin V were added to the left and middle wells, whereas the right wells received 4.8 MBq of 99mTc in the form of pertechnetate. One hour later, the cells were washed and placed on a phosphor screen, and autoradiography was performed. As seen in Figure 1, the cells without the radiosensitizer showed no significant radioactivity uptake, in stark contrast to the cells that received the photodynamic therapy. Replacing the radiolabeled annexin V with 99mTc-pertechnetate showed no retention of radioactivity. Microscopic pictures confirmed that the nonirradiated tumor cells looked healthy whereas most of the irradiated cells were in the process of undergoing apoptosis. We have observed the same behavior not only in the melanoma cancer cells shown in Figure 1 but also in A431 human epidermoid carcinoma cells and PC3 prostate cancer cells.
In vitro experiment with B16 melanoma cancer cells to detect apoptosis after photodynamic therapy. Wells B and C were incubated with the photosensitizer Pc 4, and all wells were then irradiated with laser light of 672 nm (15 kJ/cm2). One hour later, 4.8 MBq of 99mTc-annexin V was added to wells A and B, whereas well C received 4.8 MBq of 99mTc-pertechnetate. After 1 h of incubation, the wells were washed and autoradiography was performed. The cells treated with light in the absence of photosensitizer show only minor radioactivity uptake (A) and look healthy under the microscope (D). The cells treated with light in the presence of the photosensitizer show avid 99mTc-annexin V uptake (B) but no 99mTc-pertechnetate uptake (C), although their corresponding photomicrographs (E and F) show that almost every cell was undergoing apoptosis.