Abstract
(S)-11C-CGP12388 (11C-CGP12388) was recently developed as an in vivo PET tracer for the evaluation of cardiac β-adrenergic receptors. The purpose of this study was to evaluate the myocardial kinetics of 11C-CGP12388 using the perfused rat heart model. Methods: Normal rat hearts were cannulated for retrograde perfusion according to the Langendorff method. Studies were performed using constant coronary flow rates of 12 mL/min (high flow: n = 6) and 6 mL/min (low flow: n = 6). β-Adrenergic–blocking studies were also done using propranolol (blocking: n = 6). Two bolus injections of 11C-CGP12388 were administered at a 25-min interval, and time–activity curves were measured using bismuth germanate detectors. The β-adrenergic receptor density (Bmax) and total distribution volume (DVtot) were estimated using compartmental modeling. After the experiment, Bmax in vitro was measured for all hearts using 3H-CGP12177, and the values were compared with the Bmax estimated in isolated hearts. Results: DVtot was significantly lower in the blocking group than in the high-flow group (P < 0.01), and there was no significant difference in DVtot between the high- and the low-flow groups. Bmax values estimated from 11C-CGP12388 kinetics were 5.05 ± 0.90 pmol/g under the high-flow model and 5.20 ± 0.63 pmol/g under the low-flow model. The Bmax results in isolated hearts correlated significantly with the measured in vitro Bmax values (r2 = 0.69; P < 0.001). Conclusion: β-Adrenoreceptor density in the isolated rat heart can be quantified using 11C-CGP12388 and a 2-injection protocol. The binding of the tracer was flow independent, with low nonspecific binding. These results suggest that 11C-CGP12388 is a promising PET tracer that may be applicable to human studies.
Alterations of cardiac β-adrenoreceptor have been demonstrated in vitro in experimental animals or homogenates from human samples collected mainly during surgery or after death. PET with radioligands suitable for cardiac in vivo β-receptor binding studies have enabled both the imaging and the quantification of receptor density (1–3). (S)-11C-CGP12177 is a hydrophilic nonselective β-receptor antagonist that has been successfully used as a PET radiotracer for imaging cardiac β-adrenoreceptor (1,2,4–7). This radioligand is produced by reaction of 11C-phosgene with the appropriate (S)-diamine precursor in high radiochemical yield (8). The troublesome and laborious radiolabeling via 11C-phosgene is a drawback that prevents widespread use of this radioligand (3). (S)-11C-CGP12388 (11C-CGP12388), a nonselective β-adrenergic receptor ligand, was already developed as an in vivo tracer for the evaluation of β-adrenergic receptors in the heart (3). Because CGP12388 is even more hydrophilic than CGP12177, CGP12388 shares with CGP12177 the property of binding to receptors only on the myocardial cell surface and not to internalized binding sites. The biodistribution and retention of 11C-CGP12388 are reported to be similar to those of 11C-CGP12177. 11C-CGP12388 can be labeled via a 1-pot procedure using 2-11C-acetone. The synthetic procedure is easily performed and, therefore, more suitable for clinical use than is the multistep synthesis of 11C-CGP12177 (3). The purpose of the current study was to characterize the myocardial kinetics and to measure β-adrenergic receptor density (Bmax) using 11C-CGP12388 in the isolated perfused rat heart model, which has been well established as a useful model that allows control of experimental variables.
MATERIALS AND METHODS
Synthesis of 11C-CGP12388
11C-CGP12388 was synthesized at Nuklearmedizinische Klinik und Poliklinik der Technischen Universität München with a specific activity of 9.3–52 GBq/μmol. The synthesis was described in detail previously (3).
Perfusion of Isolated Rat Heart and Perfusion Protocol
Male Sprague–Dawley rats (250–350 g) were anesthetized by intraperitoneal injection of sodium pentobarbital (8–10 mg/100 g of body weight). The hearts were then quickly excised and the aorta cannulated for initiation of retrograde perfusion. Hearts were perfused with Krebs-Henseleit bicarbonate buffer (118 mmol/L NaCl, 4.7 mmol/L KCl, 2.55 mmol/L CaCl2, 1.3 mmol/L MgSO4, 1.2 mmol/L KH2PO4, and 25 mmol/L NaHCO3 containing 10 mmol/L glucose) and gassed with a mixture of 95% O2 and 5% CO2 without recirculation. A small, soft tubing for administering a bolus injection of the tracer was threaded inside the main cannula leading to the base of the aorta, just above the aortic valve. The perfused heart apparatus was heated with circulating water at a temperature of 37°C. Hearts were not externally paced.
The study was divided into 3 groups: normal flow with 12 mL/min of constant flow (high flow: n = 6; heart weight, 1.22 ± 0.18 g), slow flow with 6 mL/min (low flow: n = 5; heart weight, 1.36 ± 0.11 g), and propranolol treated with 12 mL/min (β-blocker: n = 6; heart weight, 1.29 ± 0.15 g) to assess the influence of coronary flow or β-blocker administration on CGP12388 kinetics.
The hearts with high-flow and low-flow perfusions were perfused for 15 min to allow for stabilization. The hearts in the β-blocker group were perfused with buffer containing 10 μmol/L of propranolol, and the stabilization period was extended to 25 min to ensure complete blockade of β-adrenoreceptors. In all groups, 50–70 μL (1.11–2.22 MBq) of 11C-CGP12388 were injected directly into the root of the aorta via the small tubing threaded inside the aortic cannula as the first injection after the stabilization. A second injection, containing the same radioactive dose but at 50% of the specific activity, was injected 25 min later, and the heart activity was monitored another 25 min.
After the experiment, hearts were immediately cooled in ice-cold perfusion buffer, the ventricles were cut open, and excess fluids were removed by gently blotting the heart on a paper towel. The hearts were weighed and then counted in a NaI well-counter (cpm/g of heart) to acquire detector calibration factors. To account for all radioactivity in the heart at the end of the study, the paper towel was also counted. The hearts were immediately placed into liquid nitrogen and stored at −80°C until they were processed for the in vitro measurements of β-receptor density.
Time–Activity Curves and Data Analysis
Whole-heart 11C radioactivity was measured using a pair of bismuth germanate detectors interfaced to coincidence detection circuitry built using commercially available nuclear instrumentation methods modules. Total coincidence events, random coincidence events, and the singles events of each detector were sampled and recorded every second using a personal computer. Whole-heart tissue time–activity curves were generated by subtracting random coincidence events from total coincidence events for each time point. All time–activity curves were corrected for radioactive decay.
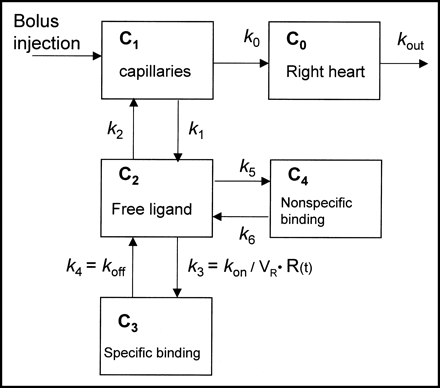
The compartmental model used in the experiment is a nonequilibrium, nonlinear model adapted for studies of the perfused rat heart (9). The model allows for the estimation of the maximal receptor density Bmax and the near equilibrium constant KD·VR, equivalent to koff divided by kon/VR. For 11C-CGP12388, the parameter VR should be close to the volume of the extracellular space in tissue (roughly equal to k1/k2 times the capillary bed volume, Vcap). The model contains 5 compartments and 8 unknown parameters (Fig. 1). The rate constant k4 is equivalent to koff of the ligand, and k3 is a time-dependent parameter equivalent to the product (kon/VR)·R(t), where R(t) is the free receptor density at time t. R(t) can be expressed by Bmax–B(t), where B(t) is the concentration of bound ligand at time t.
Compartmental model describing the kinetics of a receptor-binding radioligand administered as a bolus injection into the perfused rat heart.
The first-order rate constant k0 was fixed at k0 = F/Vcap, where F is the infusion rate (either 6 mL/min or 12 mL/min) and Vcap is the volume of the capillary bed, which was assumed to be 0.10 mL per heart (9). Thus, k0 is fixed at either 2.0 s−1 for the high-flow studies or 1.0 s−1 for the low-flow studies. Parameters were estimated using a curve-fitting program based on the Marquardt algorithm, which automatically adjusts the floated parameters to provide the best fit of the model to the time–activity data (10). Curve fitting was done using a 2-step process. First, data from the high-specific-activity injection of 11C-CGP12388 were fit to the model with k0 fixed as described above to estimate the parameters k1 to k6. For this fit, k3 was assumed to be a constant, since the high-specific-activity injection should not have led to a significant change in the free receptor density. Second, the entire 2-injection dataset was fit with k0 again fixed and with k4 (=koff) fixed to the value estimated from fitting the first injection curve alone. This second fit provided estimates of Bmax and kon for each perfused heart.
For the propranolol-blocking studies, ligand binding was assumed to be all nonspecific binding. Therefore no compartment for specific binding was used when fitting those studies (k3 = 0, k4 = 0). Total distribution volume (DVtot, in mL/g of wet weight) was calculated with parameters estimated from the first injection curve using the equation:
 where Mw is the heart wet weight (grams of wet weight per heart) and k1 to k6 have units of min−1.
where Mw is the heart wet weight (grams of wet weight per heart) and k1 to k6 have units of min−1.
In Vitro Measurement of β-Adrenoreceptor Densities
After the heart samples were thawed, they were finely dissected and minced with scissors in ice-cold Krebs-Henseleit bicarbonate buffer (pH 7.4) and homogenized with three 15-s bursts using a homogenizer (UltraTurrax; IKA Works, Inc.). The homogenate was centrifuged at 1,000g for 15 min, and the supernatant was removed and centrifuged at 100,000g for 25 min twice. The final pellet was resuspended in 50 mmol/L of Tris-HCl buffer (pH 7.4) and stored at −80°C. This was thawed and diluted to appropriate concentrations immediately before use in the assay (11).
The saturation binding assay was performed in triplicate with 3H-CGP12177 using 12.5 μmol/L propranolol to define nonspecific binding (12). In brief, a membrane suspension with a protein concentration of 0.05–0.15 mg/mL was incubated for 60 min at room temperature with various concentrations (0.01–4.0 nmol/L) of 3H-CGP12177 in a total volume of 0.25 mL of 20 mmol/L Tris-MgCl2 buffer (pH 7.4). Incubations were terminated by adding 3 mL of 50 mmol/L ice-cold Tris-HCl buffer (pH 7.4) and then using rapid vacuum filtration through GF/B filters (Whatman), followed by washing 3 times with 3 mL of the same ice-cold Tris-HCl buffer. Each filter was placed in 10 mL of scintillation cocktail and counted in a liquid scintillation counter to measure total binding and nonspecific binding. Specific binding was determined by subtracting nonspecific binding from total binding. Representative data are shown in Figures 2A and 2B. The Bmax and the equilibrium dissociation constant (KD) were determined by fitting the curve to a single binding site model using nonlinear regression (Prism; GraphPad Software, Inc.).
In vitro binding of 3H-CGP12177 to β-adrenoreceptors in membrane preparations: representative saturation binding assay curve for a heart from the group perfused at 6 mL/min (A), Scatchard plot using data from the same heart (B), and saturation binding assay curve for a heart from the propranolol-blocking group (C). No specific binding can be detected in this study.
In Vitro Kinetic Study for CGP12388
To measure the KD value of CGP12388 and compare its affinity with that of CGP12177 in vitro, competition experiments were performed in the same normal rat heart (n = 1) using 0.8 nmol/L of 3H-CGP12177 with unlabeled ligand CGP12388 and CGP12177 (0.03 nmol/L–100 nmol/L). KD values were obtained using the Cheng-Prusoff correction (13):
 where IC50 is the concentration of inhibitor that causes a 50% reduction in specific binding of the radioligand, L* is the radioligand concentration, and KD* is the equilibrium dissociation constant of the radioligand. KD* was set to the mean of the values measured for 3H-CGP12177 in the saturation binding experiments (0.95 nmol/L).
where IC50 is the concentration of inhibitor that causes a 50% reduction in specific binding of the radioligand, L* is the radioligand concentration, and KD* is the equilibrium dissociation constant of the radioligand. KD* was set to the mean of the values measured for 3H-CGP12177 in the saturation binding experiments (0.95 nmol/L).
RESULTS
Specific Activity of 11C-CGP12388 and Parameter Estimates
The specific activity of 11C-CGP12388 at the time of the synthesis was 22.2 ± 10.9 GBq/μmol (600 ± 295 mCi/μmol) in the high-flow group, 27.3 ± 19.0 GBq/μmol (738 ± 514 mCi/μmol) in the low-flow group, and 15.0 ± 10.4 GBq/μmol (406 ± 281 mCi/μmol) in the propranolol-blocking group. There was no significant difference in specific activity of the tracer between the 3 groups.
Figure 3 shows representative time–activity curves for each group. The whole-heart time–activity curve shown in Figure 3A (high-flow study) reached a maximum within the first minute after the first or the second injection. After injection, in the first minute the curve fell rapidly, then gradually decreased during the rest of the study. The curve pattern in Figure 3A is comparable to that in Figure 3B (low-flow study). In contrast, the curve in Figure 3C (β-blocking study) shows rapid decline after the initial peak, followed by a near plateau during the rest of the study.
Representative time–activity curves for each group: high-flow study (A), low-flow study (B), and β-blocker study (C). In each group, a second injection with lower specific activity was performed 25 min after the first injection. inj = injection.
Parameter estimation was successful. The estimated uncertainty of Bmax was low in all cases (uncertainty, 6.0% ± 6.0%; range, 0.1%–16.2%). Estimated kinetic parameters and β-adrenoreceptor densities are listed in Table 1. DVtot in the propranolol-blocking group was significantly lower than that in the other groups (P < 0.01). There was no statistically significant difference in koff between the groups; however, kon/VR was significantly lower in the low-flow group (P < 0.01). There was no difference in KDVR and β-adrenoreceptor density (Bmax) between the 2 groups.
Kinetic Parameters and Bmax in Isolated Rat Hearts
It was not possible to estimate the parameters Bmax, kon, and koff in the propranolol-blocking studies because specific binding was very low. However, if we assumed that 11C-CGP12388 had the same binding affinity in the propranolol-blocking study as in the high-flow studies, and if we fixed the values of kon and koff to the values obtained for the high-flow group (kon/VR = 0.094 g/min/pmol and koff = 0.24 min−1), we could then estimate Bmax = 0.0037 ± 0.0077 pmol/g in the propranolol-blocking studies. This result suggests that the infusion of 10 μmol/L propranolol blocked >99% of the receptors.
In Vitro β-Adrenoreceptor Density and KD
The in vitro cardiac β-adrenoreceptor densities (Bmax) and equilibrium dissociation constant KD were 73 ± 15 fmol/mg of protein and 0.83 ± 0.24 nmol/L, respectively, in the high-flow group and 88 ± 14 fmol/mg of protein and 1.08 ± 0.31 nmol/L, respectively, in the low-flow group. There was no significant difference in Bmax and KD between the 2 groups. In all hearts from the propanol-blocking studies, the binding assay detected no specific binding (Fig. 2C).
As shown in Figure 4, the Bmax and the KD·VR in isolated hearts measured using 11C-CGP12388 correlated with the Bmax and the KD measured by in vitro binding using 3H-CGP12177 (Bmax: r2 = 0.69 and P < 0.001; KD: r2 = 0.48 and P < 0.05).
(A) Relationship between Bmax measured in the isolated perfused heart and in vitro. (B) Relationship between KD measured in isolated hearts and in vitro.
In Vitro Kinetics of Cold CGP12388
The competition experiment with cold CGP12388 or cold CGP12177 and 3H-CGP12177 revealed IC50 values of 3.21 nmol/L for CGP12388 and 3.07 nmol/L for CGP12177. Assuming KD* = 0.95 nmol/L, KD values for the cold compounds were calculated to be 1.74 nmol/L for CGP12388 and 1.67 nmol/L for CGP12177. Thus, the KD values for the 2 compounds appear to be comparable.
DISCUSSION
To our knowledge, this is the first study in which β-adrenoreceptor densities measured in isolated rat hearts have been directly compared with values from in vitro studies. The study demonstrated that β-adrenoreceptor densities (Bmax) in isolated rat hearts can be assessed using 11C-CGP12388 with a 2-injection protocol. The binding was flow independent, with low nonspecific binding. The Bmax measured in isolated hearts correlated with the Bmax values measured in vitro.
Cardiac β-adrenoreceptor densities are reported to be altered under various conditions: for example, upregulation under severe ischemia (12,14) or sympathetic denervation (15–17) and downregulation under excessive catecholamine stimulation (18) or in cardiomyopathy (2,19–21). Direct endomyocardial biopsies have been performed to estimate cardiac β-adrenoreceptor densities in humans; however, not only is this method an invasive approach but, in addition, sampling errors frequently occur (usually only right ventricular samples). Measurement of Bmax in vivo with PET requires no direct tissue sampling and can be performed repeatedly—significant advantages over in vitro measurements of biopsy samples.
The tracer kinetic modeling approach in the present study used a standard 3-compartment receptor model with extra compartments added to account for nonspecific binding and tracer in the right heart chambers as shown in Figure 1 (9). The rate constant for specific binding k3 varies with time depending on the receptor occupancy (the higher the occupancy, the lower the k3). A single-injection protocol is unable to separately estimate receptor density (Bmax) and the association rate constant kon. After the first injection, the labeled bound ligand concentration B(t) is very low, compared with Bmax, so that we can assume that k3 = (kon/VR)·Bmax during the first injection. Therefore, the distribution volume of the tracer can be calculated with k3 from the first injection. A second injection of lower specific activity, which fills a significant fraction of available receptors, is required to estimate both kon and Bmax (1,9).
Cardiac β-adrenoreceptor densities are reported to be upregulated under severe ischemia; however, it takes hours under no flow or very low flow ischemia (<0.5 mL/min of constant flow) to establish the upregulation (12). Previous studies suggested that 5 mL/min of constant perfusion to isolated rat hearts induces no detectable overflow of creatinine kinase or lactate dehydrogenase (12). Therefore, no myocardial ischemia appears to be induced during the 6 mL/min of constant flow used in the low-flow conditions in the present study. Hemodynamic and physiologic conditions in the isolated hearts may influence the tracer kinetics. Preliminary experiments to measure hemodynamic parameters during perfusion studies were performed under various flow conditions. The results showed that coronary perfusion pressure correlated linearly with constant flow rate. Coronary perfusion pressure in hearts perfused at 6 mL/min was about half that in hearts perfused at 12 mL/min (33 ± 5.5 mm Hg vs. 67 ± 16 mm Hg), and HR in the 6 mL/min hearts was around 7% lower than that in hearts perfused at 12 mL/min (242 ± 32 beats per minute vs. 261 ± 25 beats per minute). Physiologic alterations may induce significant reductions in the kon/VR value. However, the Bmax data were not different between the low-flow and high-flow groups, a finding that is reasonable because the altering flow did not induce any pathophysiologic conditions.
Our results indicated that the binding affinity KD and receptor density Bmax estimated in the high- and the low-flow groups were equivalent. The results suggest that estimated parameters in isolated hearts are flow independent under nonischemic conditions.
The in vitro procedure for measuring Bmax in this study measures receptor densities only on the cell membrane, expressed in units of fmol/mg of protein. Although Bmax in isolated hearts was expressed in units of pmol/g of wet weight, 11C-CGP12388 is a hydrophilic tracer that binds to β-receptors expressed on the cell membrane. Therefore, the estimated Bmax in isolated hearts theoretically should reflect the in vitro Bmax measurement. The significant correlation between the KD and Bmax values estimated in isolated hearts and the corresponding in vitro measurements of these parameters suggests that the 2-injection method in isolated hearts can substitute for the conventional in vitro method.
Many radiolabeled β-adrenoreceptor ligands have been studied for in vivo measurement of the β-adrenoreceptor density (22–26). Most of them, however, demonstrated unsatisfactory properties for the assessment of cardiac β-adrenoreceptors (22,23). 125I- and 123I-iodocyanopindolol exhibit low nonspecific binding and extremely high affinity for the receptor (KD = 0.018 nmol/L for β1 and 0.02 nmol/L for β2) (23) and thus were potentially good candidates for the in vivo evaluation of cardiac β-adrenoreceptors (24). However, the fact that iodocyanopindolol shows high uptake in the lungs (24) is a major drawback for cardiac imaging studies. In addition, because of its moderately high lipophilicity (log P = +1.26) (23), the tracer binds to internalized β-receptors as well as to cell surface receptors. A more hydrophilic tracer is more suitable for the evaluation of cardiac β-adrenoreceptors since it will assess only the functional receptors expressed on the cell surface.
11C-CGP12177 has frequently been used for the evaluation of cardiac β-adrenoreceptors in vivo (1,2,4,6,7) because of its hydrophilicity (log P = −0.50) and its relatively high binding affinity (KD = 0.33 nmol/L for β1 and 0.90 nmol/L for β2) (27). The compound is an ideal tracer for tracer kinetics analysis. The fact that the kinetics of both CGP compounds are similar and 11C-CGP12388 is easier to synthesize than 11C-CGP12177 makes 11C-CGP12388 an attractive alternative to 11C-CGP12177 for assessing cardiac β-adrenoreceptors with PET.
Development of a β1-subtype selective radioligand would be a significant advance for cardiac PET studies of β-adrenergic receptor populations. 11C-CGP20712A, a β1-selective antagonist, was synthesized and evaluated in animal studies; however, this radioligand exhibited very high nonspecific binding (26). A future direction for this area would be to focus on radioligands possessing subtype selectivity for β-adrenergic receptors.
In applications of kinetic modeling to human studies, metabolite formation and plasma binding activity need to be considered. Elsinga et al. (28) reported that for 5 healthy volunteers, 87% of the radioactivity in plasma for this ligand was still unchanged radioligand at 10 min after injection. However, plasma clearance of 11C-CGP12388 after intravenous injection was rapid and was well described by 3 exponential decay processes with half-times of 0.22, 2.51, and 101.5 min (28). These results indicate that plasma binding and radiolabeled metabolites should be negligible. Therefore, kinetic modeling of data from a 2-injection protocol, as used in our studies in the isolated rat heart, should be applicable to human studies. In fact, the Groningen group has also reported the first series of human 11C-CGP12388 PET studies, in which a 3-compartment model was successfully applied to estimation of Bmax and other model parameters (29).
A limitation of this ligand, as well as of other β-receptor ligands used in vivo, is an underestimation of β-adrenoreceptor densities under β-blocking conditions. An assessment of β-adrenoreceptors for human studies therefore requires complete withdrawal of the β-blocking agent if a patient is taking the medication.
CONCLUSION
Cardiac β-adrenoreceptor density in the isolated rat heart could be quantified using 11C-CGP12388 and a 2-injection protocol. The binding was flow independent and highly specific. These results indicate that 11C-CGP12388 is a promising PET tracer suitable for human studies.
Acknowledgments
This work was supported by grants from Deutsche Forschungs Gemeinschaft. The authors thank Jodi Neverve for her careful editing.
Footnotes
Received May 27, 2003; revision accepted Oct. 23, 2003.
For correspondence or reprints contact: Markus Schwaiger, MD, Nuklearmedizinische Klinik und Poliklinik der Technischen Universität München, Klinikum rechts der Isar, Ismaninger Strasse 22, 81675 Munich, Germany.
E-mail: M.Schwaiger{at}lrz.tum.de