Abstract
131I-Iodide is the treatment of choice in most cases of hyperthyroidism, with a standard 7,000-cGy (rad) thyroid absorbed dose generally resulting in an incidental blood absorbed dose of less than 10 cGy (rad). However, in ∼15% of patients there is a small, rapidly secreted thyroid iodine pool (small-pool patients) and, based on theoretic calculations, an incidental blood absorbed dose of up to 150 cGy (rad) could result. In such small-pool patients, continuing antithyroid drugs (ATDs) at a reduced dosage during 131I therapy should inhibit the formation of 131I-labeled levothyroxine and triiodothyronine and thereby reduce the protein-bound 131I-iodine concentration in blood and the blood absorbed dose. Methods: To test this hypothesis, thyroid and blood time–activity data were measured and absorbed doses were calculated for an 131I tracer administered to small-pool hyperthyroid patients (n = 9) not receiving ATDs (off ATDs) and then receiving ATDs (on ATDs). Results: The blood absorbed dose (cGy/37 MBq [rad/mCi] administered) was reduced from 2.54 ± 0.91 (mean ± SD) without ATDs to 1.27 ± 0.54 with ATDs (P < 0.0001), whereas the thyroid absorbed dose was unchanged (1,870 ± 700 vs. 2,080 ± 1,080). The blood absorbed dose for an administered 131I activity required to deliver a standard prescribed absorbed dose of 7,000 cGy (rad) to the thyroid therefore was reduced by over 50% with ATDs, from 11.3 ± 6.5 to 4.9 ± 2.8 cGy (rad) (P < 0.001). Conclusion: Continued administration of ATDs during 131I therapy thus can effectively reduce extrathyroid radiation in small-pool patients without significantly reducing the target tissue (i.e., thyroid) dose.
Optimal radioiodine (131I-iodide) treatment of hyperthyroidism requires the delivery of an appropriate radiation-absorbed dose to the thyroid gland. Extrathyroid radiation from administered 131I is inevitable but is assumed to be minimal and inconsequential and is rarely measured anymore. Common follow-up protocols may examine thyroid function for a limited period of time for signs of hypothyroidism, but even that procedure is considered onerous by busy clinicians who increasingly prefer to deliberately ablate the thyroid and immediately place the patient on thyroid hormone replacement (supplement). Possible other late consequences of incidental whole-body radiation usually are ignored. It is particularly important therefore to administer radioiodine therapy in such as way as to minimize extrathyroid irradiation and the possibility of long-term radiogenic sequelae while still delivering a therapeutically appropriate target tissue (i.e., thyroid) dose.
Besides radioiodine uptake and thyroid mass, the thyroid radiation dose is determined by the residence time (biologic half-time) of the radioiodine within the thyroid gland (1–3). In euthyroid individuals, the biologic half-time of radioiodine in the thyroid is ∼90 d (range, 60–120 d). In contrast, hyperthyroid patients generally discharge radioiodine more rapidly, with an average biologic half-time of only 30 d (range, 10–40 d). The majority of the radioiodine leaves the thyroid as radiolabeled levothyroxine (T4) and triiodothyronine (T3), which bind to serum proteins. They have a prolonged residence time in the circulation and are responsible for as much as 90% of the blood and whole-body radiation-absorbed dose. The levels of circulating radioactive thyroid hormones can be quantified by determining the serum protein–bound 131I (PB131I) concentration. As previously reported (4), ∼15% of hyperthyroid patients have accelerated thyroid turnover of radioiodine, thought to be attributable to a small thyroid iodine pool (small-pool syndrome) and perhaps reflecting very high levels of thyroid stimulation. As a result, the PB131I concentration can be markedly elevated, and the absorbed doses to blood and other extrathyroid tissues can be substantially higher than those usually seen. In this study, a small-pool patient was operationally defined as a hyperthyroid patient in whom at least 1 PB131I measurement when the patient was not receiving antithyroid drugs (ATDs) (off ATDs) was greater than 2% of the administered activity per liter.
In addition, the relatively small thyroid absorbed dose in small-pool patients likely is responsible for at least some radioiodine treatment failures whenever the administered radioiodine is based on a standard administered activity or any other method that does not take into account the measured thyroid turnover rate. For example, a standard thyroid absorbed dose of 7,000 cGy requires only 222–259 MBq (6–7 mCi) of 131I in typical hyperthyroid patients but requires ∼333 MBq (9 mCi) in small-pool patients. Barandes et al. previously reported a series of 7 small-pool hyperthyroid patients in whom a standard thyroid absorbed dose of 7,000 cGy would require 1,036 MBq (28 mCi) of 131I and theoretically would yield a radiation dose in the blood of 150 cGy (4), a prohibitively high incidental radiation dose for the treatment of a benign disease. In contrast, the usual blood absorbed dose for hyperthyroid patients is less than 10 cGy.
An approach was therefore devised to decrease the release of radiolabeled T3 and T4 and thus lower the PB131I concentration by continuing a low dosage of ATDs throughout the period of radioiodine treatment. It is the usual practice in the treatment of hyperthyroid patients receiving ATDs (on ATDs), such as propylthiouracil or methimazole, to discontinue these medications 2–5 d preceding 131I therapy, presumably to avoid interfering with thyroid radioiodine uptake (5). ATDs also have been reported to accelerate radioiodine turnover (6). However, ATDs do not affect the iodine-trapping mechanism (and therefore radioiodine uptake) and do not interfere with the initial binding of iodine to tyrosine residues on thyroglobulin (organification) unless given in large dosages. On the other hand, coupling of 3-monoiodotyrosine and 3,5-diiodotyrosine to form T4 and T3 is sensitive to even low concentrations of ATDs (7). In small-pool patients, therefore, continuation of ATDs during radioiodine therapy should reduce the PB131I concentration and the blood radiation dose without significantly reducing the thyroid radiation dose. Potentially, most (i.e., typical) hyperthyroid patients, in whom the ratio of the thyroid absorbed dose to the blood absorbed dose is already quite favorable (especially in uncomplicated Graves’ disease), would also benefit from the continuation of ATDs through the maintenance of uninterrupted symptomatic control. Thus, continued administration of ATDs during 131I therapy should markedly reduce extrathyroid radiation in small-pool patients and should maintain symptomatic control without adversely affecting the ratio of the thyroid absorbed dose to the blood absorbed dose. The purpose of this study was to perform a dosimetric analysis of 131I in small-pool hyperthyroid patients off ATDs and on ATDs and thereby to test this hypothesis.
MATERIALS AND METHODS
In our laboratory, hyperthyroid patients are treated with radioiodine according to a prescribed absorbed-dose algorithm, with the following general guidelines for definitive single-dose therapy (1,8): for young patients and those with uncomplicated Graves’ disease (i.e., small glands and mild-to-moderate hyperthyroidism), 7,000–8,000 cGy; for patients with complicated Graves’ disease (i.e., larger glands and more severe hyperthyroidism), 10,000–12,000 cGy; and for patients with toxic nodular goiter, 15,000–25,000 cGy. It is important to note that there is substantial evidence that ATDs administered before, with, or after radioiodine therapy induce radioresistance, necessitating an administered activity of radioiodine higher than usual (9). In order to estimate the absorbed dose per unit of administered 131I activity in the thyroid and thus the therapeutic administered activity for the prescribed thyroid dose, all hyperthyroid patients considered for radioiodine treatment in our laboratory were first studied after the oral administration of a 1.11-MBq (30 μCi) 131I tracer. Thyroid uptake and blood activity concentrations (in serum and whole blood and as the PB131I concentration) generally were measured 3 or 4 times from 1 to as late as 7 d after the administration of the radioiodine tracer (1–3). In several instances, however, only 2 PB131I measurements were obtained, with the last measurement at 2 d after administration.
Thyroid uptake (percentage of administered activity) was measured at a skin-to-aperture distance of 30 cm using a “thyroid” uptake probe fitted with an open-bore collimator and set to an 131I photopeak energy window of 364 keV ± 10%, subtracting a thigh background counting rate from the gross neck counting rate and comparing the resulting net neck counting rate to the net counting rate for a standard containing a known fraction of the administered 131I activity. The net counting rate for the standard was calculated by subtracting the “room background” counting rate, that is, the counting rate without either the patient or the standard in the room, from the gross counting rate for the standard. Blood and serum activity concentrations were determined from measured volumes of blood and serum, respectively, in a scintillation well counter also set to an 131I photopeak energy window of 364 keV ± 10% and comparing the resulting net blood sample counting rate to the net counting rate for a standard of equal volume and with a known dilution of the administered 131I activity. The PB131I concentration was determined by molecular exclusion column chromatography of a serum sample.
This study, restricted to Graves’ disease, included 9 patients found to have small-pool hyperthyroidism, defined on the basis of at least 1 PB131I measurement that was greater than 2% of the administered activity per liter for patients off ATDs (5). Although many of these patients had been treated for prolonged periods with ATDs, none had received ATDs for at least 1 wk preceding the 131I tracer study. ATDs were resumed at a dosage one third to one half the previous maintenance dosage. The patients were reevaluated at 2-wk intervals, and the ATD dosage was adjusted to maintain serum T3 and T4 at the upper limit of normal. The ATD dosage required to maintain serum T3 and T4 at this level was individually determined for each patient, requiring repeat measurements over at least a 4-wk period. Once this level was achieved, a repeat radioiodine tracer study was performed and radioiodine therapy was administered within 1 wk thereafter. ATDs were maintained at this same dosage throughout the period of the tracer and therapy administrations and for at least 1 wk thereafter. (In nearly all cases, the morning dose of ATD was omitted on the days on which the tracer 131I and the therapy 131I were administered.)
The serial thyroid uptake measurements were fit to a monoexponential function with the numeric module of the SAAM II program (10–12), and the cumulated activity of 131I in the thyroid was calculated by incorporation of physical decay and integration of the resulting function, as follows:
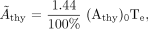 Eq. 1 where Ãthy is the cumulated activity in the thyroid per unit of administered 131I activity, (Athy)0 is the fitted zero-time thyroid uptake (percentage of administered activity), and Te is the effective half-time of 131I in the thyroid, expressed by the following equation:
Eq. 1 where Ãthy is the cumulated activity in the thyroid per unit of administered 131I activity, (Athy)0 is the fitted zero-time thyroid uptake (percentage of administered activity), and Te is the effective half-time of 131I in the thyroid, expressed by the following equation:
 Eq. 2 where Tb is the fitted biologic half-time of 131I in the thyroid and Tp is the physical half-life of 131I, that is, 8.04 d.
Eq. 2 where Tb is the fitted biologic half-time of 131I in the thyroid and Tp is the physical half-life of 131I, that is, 8.04 d.
Thyroid absorbed doses then were calculated by use of the MIRD formulation developed by the MIRD Committee of the Society of Nuclear Medicine (2,3). The total thyroid absorbed dose was equated with its self-irradiation absorbed dose (2,3), as follows:
 Eq. 3
Eq. 3
 Eq. 4 where D̅(thy) is the mean absorbed dose in the thyroid per unit of administered 131I activity, D̅(thy ← thy) is the mean self-irradiation absorbed dose in the thyroid per unit of administered 131I activity, and S(thy ← thy) is the thyroid-to-thyroid S factor, that is, the mean absorbed dose to the thyroid per unit cumulated activity in the thyroid, and given by the following expression (13):
Eq. 4 where D̅(thy) is the mean absorbed dose in the thyroid per unit of administered 131I activity, D̅(thy ← thy) is the mean self-irradiation absorbed dose in the thyroid per unit of administered 131I activity, and S(thy ← thy) is the thyroid-to-thyroid S factor, that is, the mean absorbed dose to the thyroid per unit cumulated activity in the thyroid, and given by the following expression (13):
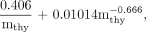 Eq. 5 where mthy is the thyroid mass (g), as estimated by palpation.
Eq. 5 where mthy is the thyroid mass (g), as estimated by palpation.
The cumulated activity concentration of 131I in the blood was calculated by numeric integration of the serial blood time–activity concentration measurements, with the assumptions of elimination by physical decay only after the last measurement (with analytic integration of this terminal portion) and the incorporation of physical decay (2,14), as follows:
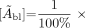 Eq. 6
Eq. 6 where [Ãbl] is the cumulated activity concentration in blood per unit of administered 131I activity, [Abl]i is the activity concentration in blood (percentage of administered 131I activity per milliliter) at time after administration ti, and [Abl]0 is the zero-time activity concentration in blood (percentage of administered 131I activity per milliliter), which is expressed by the following equation:
where [Ãbl] is the cumulated activity concentration in blood per unit of administered 131I activity, [Abl]i is the activity concentration in blood (percentage of administered 131I activity per milliliter) at time after administration ti, and [Abl]0 is the zero-time activity concentration in blood (percentage of administered 131I activity per milliliter), which is expressed by the following equation:
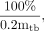 Eq. 7 where mtb is the total body mass (g).
Eq. 7 where mtb is the total body mass (g).
Blood absorbed doses then were calculated, again by use of the MIRD formulation, as the sum of the blood self-irradiation absorbed dose and the thyroid-to-blood absorbed dose; this strategy assumes, reasonably, that the only significant “non-self” dose contribution to blood arises from the thyroid. The blood self-irradiation absorbed dose was calculated with the assumption of complete local absorption of nonpenetrating radiation (i.e., 131I β-rays) only. The thyroid (thy)-to-blood (bl) absorbed dose was calculated by approximating the thyroid-to-blood S factor (i.e., the mean absorbed dose to the blood per unit cumulated activity in the thyroid) as the Standard Man thyroid (thy)-to-muscle (mus) S factor (i.e., the mean absorbed dose to the muscle per unit cumulated activity in the thyroid) (2,15–17), since both blood and muscle are similarly (i.e., widely) distributed tissues:
 Eq. 8
Eq. 8
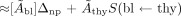 Eq. 9
Eq. 9
 Eq. 10 where D̅(bl) is the total mean absorbed dose to the blood per unit of administered 131I activity, D̅np(bl ← bl) is the mean absorbed dose to blood specifically from nonpenetrating (np) radiations (i.e., β-rays) emitted from 131I in the blood per unit of administered 131I actitivy, D̅(bl ← thy) is the mean absorbed dose to blood from 131I in the thyroid per unit of administered 131I activity, Δnp is the equilibrium dose constant for nonpenetrating radiation for 131I, that is, 0.405 g-Gy/37 kBq-h (g-rad/μCi-h) (18), and S(mus ← thy) is the Standard Man thyroid-to-muscle S factor, that is, 3.8 × 10−6 cGy/37 kBq-h (rad/μCi-h) (17).
Eq. 10 where D̅(bl) is the total mean absorbed dose to the blood per unit of administered 131I activity, D̅np(bl ← bl) is the mean absorbed dose to blood specifically from nonpenetrating (np) radiations (i.e., β-rays) emitted from 131I in the blood per unit of administered 131I actitivy, D̅(bl ← thy) is the mean absorbed dose to blood from 131I in the thyroid per unit of administered 131I activity, Δnp is the equilibrium dose constant for nonpenetrating radiation for 131I, that is, 0.405 g-Gy/37 kBq-h (g-rad/μCi-h) (18), and S(mus ← thy) is the Standard Man thyroid-to-muscle S factor, that is, 3.8 × 10−6 cGy/37 kBq-h (rad/μCi-h) (17).
The statistical significance of differences in 131I kinetic and dosimetric parameters between patients who were off ATDs and those who were on ATDs was evaluated by use of the Student paired t test; a P value of 0.05 or less was considered statistically significant (19).
RESULTS
The measured 131I-iodide tracer kinetics and the resulting blood and thyroid radiation dosimetry data for the 9 small-pool hyperthyroid patients in this study are shown in Tables 1 and 2, respectively. These data are also summarized graphically, with the results of statistical testing, in Figures 1–6.
Decay-corrected concentration (percentage of administered activity per liter of serum) of PB131I at 24 h after administration of 131I-iodide tracer in 9 small-pool hyperthyroid patients either off or on ATDs at the time of radioiodine administration. Numeric values and errors bars represent mean ± SD. P value represents the result of the Student paired t test of the statistical significance of the difference between the 24-h PB131I concentrations in patients off ATDs and on ATDs; difference was statistically significant at a 0.1% significance level.
131I-Iodide Tracer Kinetics in Small-Pool Hyperthyroid Patients Off and On ATDs
131I-Iodide Dosimetry in Small-Pool Hyperthyroid Patients Off and On ATDs
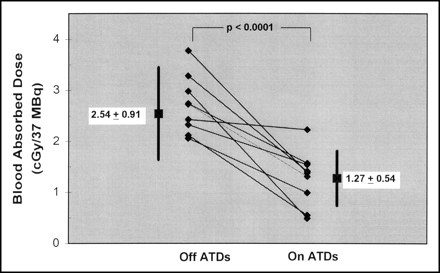
In small-pool hyperthyroid patients, continuation of ATDs during 131I administration markedly reduced the PB131I concentration (Fig. 1), with little or no effect on thyroid uptake (Fig. 2) but with a small but significant prolongation of the biologic half-time (Fig. 3). As a result, expressed in terms of absorbed dose per unit of administered 131I activity, there was a substantial reduction in the blood absorbed dose (Fig. 4) but no significant change in the thyroid absorbed dose (Fig. 5). Perhaps most importantly, there was likewise a substantial reduction in the blood absorbed dose, ranging from 37% to 68% (Table 2), for the administered 131I activity required to deliver a standard prescribed absorbed dose of 7,000 cGy to the thyroid (Fig. 6).
Decay-corrected thyroid uptake (percentage of administered activity) at 24 h after administration of 131I-iodide tracer in 9 small-pool hyperthyroid patients either off or on ATDs at the time of radioiodine administration. Numeric values and errors bars represent mean ± SD. P value represents the result of the Student paired t test of the statistical significance of the difference between the levels of thyroid uptake at 24 h in patients off ATDs and on ATDs; difference was not statistically significant (NS) at a 5% significance level.
Biologic half-time (T1/2) in thyroid of 131I-iodide tracer administered to 9 small-pool hyperthyroid patients either off or on ATDs at the time of radioiodine administration. Numeric values and errors bars represent mean ± SD. P value represents the result of the Student paired t test of the statistical significance of the difference between the biologic half-times in patients off ATDs and on ATDs; difference was statistically significant at a 5% significance level.
Calculated mean absorbed dose to blood (cGy/37 MBq [rad/mCi] of administered activity) from 131I-iodide tracer in 9 small-pool hyperthyroid patients either off or on ATDs at the time of radioiodine administration. Numeric values and errors bars represent mean ± SD. The P value represents the result of the Student paired t test of the statistical significance of the difference between the blood absorbed doses in patients off ATDs and on ATDs; difference was statistically significant at a 0.01% significance level.
Calculated mean absorbed dose to thyroid (cGy/37 MBq [rad/mCi] of administered activity) from 131I-iodide tracer in 9 small-pool hyperthyroid patients either off or on ATDs at the time of radioiodine administration. Numeric values and errors bars represent mean ± SD. P value represents the result of the Student paired t test of the statistical significance of the difference between the thyroid absorbed doses in patients off ATDs and on ATDs; difference was not statistically significant (NS) at a 5% significance level.
Calculated mean absorbed dose to blood for the administered 131I-iodide activity required to deliver a standard prescribed absorbed dose of 7,000 cGy, based on measured tracer kinetics, in 9 small-pool hyperthyroid patients either off or on ATDs at the time of radioiodine administration. Numeric values and errors bars represent mean ± SD. P value represents the result of the Student paired t test of the statistical significance of the difference between the blood absorbed doses in patients off ATDs and on ATDs; difference was statistically significant at a 0.1% significance level.
DISCUSSION
The clinical effectiveness of radioiodine treatment of hyperthyroidism is presumably related to the absorbed dose to thyroid follicular cells. However, the most common dose prescription algorithm, a fixed administered activity, entails the administration of a specified activity (at this time, typically on the order of 370 MBq [10 mCi]) to all hyperthyroid patients. If the highly variable individual parameters of thyroid uptake, biologic half-life, and mass, which determine absorbed dose, are ignored, it is unlikely that there will be a correlation between the administered activity and the absorbed dose (2,20). Although up to 85% of patients will eventually be cured (i.e., made euthyroid or hypothyroid) by the administration of single doses of standard amounts of radioiodine (and presumably 100% with repeated administrations) (1,8,21), such an arbitrary approach does not permit individually optimized therapy and would not identify patients, such as small-pool patients, for whom radioiodine therapy may be inappropriate or even hazardous (4,5).
Barandes et al. previously reported a series of 7 small-pool hyperthyroid patients in whom a standard 7,000-cGy absorbed dose would require 1,036 MBq (28 mCi) of 131I and theoretically would yield a radiation dose to the blood of 150 cGy (4), a prohibitively high incidental radiation dose for the treatment of a benign disease. Interestingly, the 131I blood absorbed dose among these small-pool patients, 5.3 cGy/37 MBq, was 2-fold higher than that among the small-pool patients who were off ATDs in the present study, 2.5 cGy/37 MBq; this finding was attributable to a 2-fold difference in the maximum PB131I concentration between these 2 groups of patients. However, the absence of a significant difference in any kinetic or dosimetric parameter between the “typical” (non-small-pool) patients in the earlier study (4) and the small-pool patients on ATDs in the present study suggests that these 2-fold differences in the PB131I concentration and the 131I blood absorbed dose were not related to any methodologic differences between the 2 studies but rather were simply random variations between small groups of patients.
In previously reported attempts to circumvent this problem (4), potassium iodide supplements given before radioiodine treatment decreased the 24-h PB131I concentration from 4.4%/L to 1.7%/L and increased the biologic half-time of radioiodine in the thyroid from 2.8 to 6.5 d. Although this regimen was successful, problems in logistics and compliance made the regimen difficult to implement. In addition, 1 patient had an exacerbation of hyperthyroidism, with an impending thyroid storm, and another had a slight increase in symptoms of hyperthyroidism.
The results of this study clearly indicate that continuation of low-dosage ATDs throughout the period of radioiodine treatment substantially reduces (typically by more than 50%) the blood absorbed dose in small-pool patients without a significant reduction in the target tissue (i.e., thyroid) absorbed dose. Consistent with our hypothesis, low-dosage ATDs appear to reduce the secretion of 131I-labeled T4 and T3, as indicated by the substantially reduced 24-h PB131I concentration (Fig. 1). At the same time, ATDs, at least at low dosages, do not appear to affect the uptake of iodine by the thyroid, as indicated by the unchanged 24-h thyroid uptake (Fig. 2), and actually result in a slightly longer biologic half-time for radioiodine in the thyroid (Fig. 3). When the administered activity needed to deliver a prescribed radiation-absorbed dose to the thyroid is based on an individualized calculation, an appropriate, curative radiation dose can be delivered.
Although the number of patients in this study, 9, was small, the results are nonetheless compelling, because each patient served as his or her own control. That is, the 131I tracer kinetics and dosimetry data for each patient on ATDs were compared with those for the same patient off ATDs, and the statistical significance of any changes in kinetic and dosimetric parameters was evaluated by use of the paired t test.
CONCLUSION
In summary, continuation of low-dosage ATDs throughout the period of radioiodine treatment substantially reduces the extrathyroid radiation dose in small-pool patients, in whom radioiodine treatment arguably would otherwise be inappropriate on a risk-benefit basis, without a significant reduction in the thyroid absorbed dose. Moreover, continuation of low-dosage ATDs provides the important clinical advantage of uninterrupted symptomatic control.
Footnotes
Received Jul. 20, 2004; revision accepted Aug. 11, 2004.
For correspondence contact: Pat B. Zanzonico, PhD, Memorial Sloan-Kettering Cancer Center, 1275 York Ave., New York, NY 10021.
E-mail: zanzonip{at}mskcc.org