Abstract
The immunosuppressive agent FK506 (tacrolimus) has neuroprotective properties not only in rodents but also in nonhuman primates. To improve the accuracy of clinical studies of acute stroke, clinical dose setting based on brain concentrations of agents in humans is very helpful. We have already established a rapid-synthesis method for 11C-labeled FK506; therefore, in the present study, we aimed to establish a method to measure brain concentrations of FK506 using 11C-FK506 PET in monkeys. Methods: Studies were performed on 3 male cynomolgus monkeys (Macaca fascicularis). FK506 (0.1 mg/kg) containing 11C-FK506 was intravenously injected into the monkeys, and dynamic PET images were acquired for 30 min afterward. Arterial blood samples were collected 5 and 15 min after injection, and their radioactivities were measured by a γ-counter. FK506 concentrations in brain and blood were calculated in units of moles per liter using the specific activity of the injected FK506. The PET study data were validated using an enzyme-linked immunosorbent assay. Results: Seven minutes after administration, the radioactivity in the brain became constant and was maintained up to 30 min. We succeeded in measuring the FK506 concentration in the brain using 11C-FK506 PET. Fifteen minutes after FK506 (0.1 mg/kg) administration, the concentrations in the cortex and striatum were 20.0 ± 1.7 ng/g and 14.1 ± 1.7 ng/g, respectively. FK506 concentrations in the blood correlated significantly with those measured by enzyme-linked immunosorbent assay. Conclusion: We successfully measured FK506 concentrations in anesthetized monkey brain and blood using 11C-FK506 PET. These results indicate a potential method to measure FK506 concentrations in human brain. Additionally, a potential use for the PET technique in drug development has been demonstrated.
In drug development, the transition from preclinical studies to the clinic is a critical step. In the realm of neuroprotective drugs, which aim to salvage ischemic tissue, limit infarct size, or minimize postischemic reperfusion injury, good results have been shown in preclinical testing. However, these compounds have not ultimately been successful (1,2). One reason for the failure of so many trials may be discrepancies between efficacious dose and toxicity between animal and clinical studies. It is of course expected that drugs will differ in effectiveness and pharmacokinetics between humans and experimental animals. Clinical dose setting is therefore one of the most essential steps in translating the results of animal studies into clinical trials. It is important to estimate the neuroprotective properties of agents using nonhuman primate models of stroke and to set the clinical dose on the basis of effective drug concentrations in the human brain. The blood-brain barrier makes it difficult to estimate the therapeutic dose, so that delivery of drug to the brain and brain concentrations should be confirmed after injection of the effective dose. Currently, to measure brain concentrations of agents in humans, PET represents the only viable method.
The immunosuppressive agent FK506 (tacrolimus) is well known to have neuroprotective properties (3–7). The neuroprotective effects of this agent have been demonstrated in rodents (3–6) and also in a nonhuman primate model of focal cerebral ischemia (7). FK506 has also been reported to be a substrate of P-glycoprotein in the blood-brain barrier, which is a transmembrane drug efflux pump (8). We have already reported a rapid-synthesis method for 11C-labeled FK506 using an automated apparatus (9). Therefore, in the present study, we aimed to establish a method for measuring FK506 concentrations in the brain and blood of monkeys using 11C-FK506 PET. The data from the PET study were validated using a published method for FK506 measurement. The brain concentration data may facilitate the prediction of neuroprotective concentrations in humans.
MATERIALS AND METHODS
Animal Preparation
These studies were performed on 3 male cynomolgus monkeys (Macaca fascicularis) with body weights ranging from 5.0 to 7.0 kg (Clea Japan Inc.). Monkeys were maintained and handled in accordance with the recommendations of the U.S. National Institutes of Health, and all animal experiments were performed in compliance with the regulations of the animal ethics committee of the Medical and Pharmacologic Research Center Foundation. Monkeys were anesthetized with intramuscular ketamine hydrochloride (10 mg/kg), tracheostomized, immobilized with intramuscular pancuronium bromide (0.05 mg/kg) every 2 h, and artificially ventilated. Anesthesia was continued with isoflurane (0.1%–0.4%) in a 7:3 N2O:O2 gas mixture during the entire experiment. The right saphenus artery was catheterized for measurement of arterial blood pressure and heart rate and for sampling of arterial blood. The left saphenus vein was also catheterized for injection of 11C-FK506 and FK506. The mean arterial blood pressure, heart rate, rectal temperature, arterial partial pressures of oxygen and carbon dioxide, pH, and plasma glucose levels were continuously or regularly monitored.
Drug Preparation
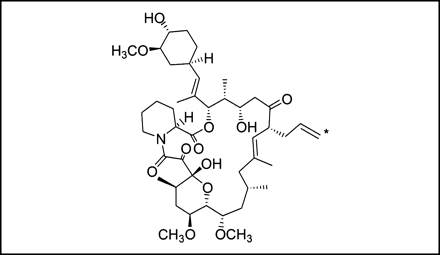
Before each study, an automated apparatus synthesized 11C-FK506 (Fig. 1) as a solution in 2.5 mL of 33% ethanol-saline. This solution was diluted with saline to 10% ethanol-saline. FK506 (10 mg/mL; Fujisawa Pharmaceutical Co. Ltd.) in polyoxyethylene hydrogenated castor oil 60 (400 mg/mL) and ethanol was diluted with sterilized saline, and a 0.2 mg/mL solution was made. To this solution, the same volume of 11C-FK506 saline solution containing 10% ethanol was added and mixed. The final concentration of FK506 was 0.1 mg/mL in 10% ethanol-saline. In this preparation, the amount of 11C-FK506 was calculated to be less than 1% of the total amount of FK506.
Chemical structure of 11C-FK506. Asterisk denotes position of 11C labeling.
Drug Administration
FK506 containing 11C-FK506 (132–239 MBq) was administered intravenously at a dose of 0.1 mg/kg (1.0 mL/kg) over 60 s via the catheter in the left saphenus vein.
PET Experiment
PET scans were obtained with a high-resolution animal PET scanner (SHR7700; Hamamatsu Photonics) with a transaxial resolution of 2.6 mm in full width at half maximum and a center-to-center distance of 3.6 mm (10). The PET camera allowed 31 imaging slices to be recorded simultaneously. A 68Ge-68Ga blank scan (120 min) was obtained before each study. Each monkey’s head was fixed in a plastic stereotactic apparatus (SFCT-RB-2; Hamamatsu Photonics) that had already been fixed in the PET scanner gantry, and a 68Ge-68Ga-transmission scan (30 min) was then obtained before PET measurement. A 30-min emission scan was obtained after injection of 11C-FK506. The measurement time frames were as follows: 10 s × 18 frames, 1 min × 7 frames, and 5 min × 4 frames. Five and 15 min after the start of the FK506 injection, arterial blood samples were collected via the arterial catheter using heparinized syringes. Whole-blood radioactivity was measured by a γ-counter (1480 WIZARD; Wallac Oy). PET images were anatomically standardized using Neurostat (University of Michigan) (11), and regions of interest were taken on the images of cerebral cortex and striatum. The data obtained from the γ-counter and PET scans, in units of becquerels per milliliter, were converted to units of nanograms per milliliter according to the calculated specific activity of the 11C-FK506 preparation for each animal. Brain concentrations were calculated after correction for the effect of cerebral blood volume. Here, the radioactivity corresponding to cerebral blood volume was assumed to be 3% of whole-blood radioactivity. All data are represented as mean ± SD.
Measurement of FK506 Concentration in Whole Blood
Collected whole-blood samples were immediately cooled to 4°C and then frozen at −80°C until being analyzed for FK506 by enzyme-linked immunosorbent assay (ELISA). The FK506 concentration in whole blood was measured by the ELISA method published by Tamura et al. (12).
RESULTS
A typical PET image set acquired from 10 to 30 min after administration of 11C-FK506, and the corresponding MR image set, are shown in Figure 2. The time courses of the FK506 concentration in the cortex and striatum are shown in Figure 3. The FK506 concentration in the brain increased immediately after injection, became constant 7 min after injection, and then was maintained up to 30 min. The concentrations in brain and whole blood are shown in Table 1.
Typical PET image set acquired from 10 to 30 min after administration of FK506 (0.1 mg/kg) containing 11C-FK506, and corresponding MR image set. The images were anatomically standardized using Neurostat (University of Michigan) and are transverse slices at 6 levels from the anterior commissure-posterior commissure (AC-PC). Leftmost slice is AC-PC + 10 mm and rightmost slice is AC-PC − 5 mm, with 3 mm between slices.
Time-activity curves in cortex and striatum after administration of FK506 (0.1 mg/kg) containing 11C-FK506. Each symbol represents mean of 3 animals, and bar represents SD.
PET-Determined FK506 Concentrations After 0.1 mg/kg Intravenous Administration of 11C-FK506 to Anesthetized Monkeys
In these experiments, the physiologic parameters of the monkeys (mean arterial blood pressure, heart rate, rectal temperature, arterial partial pressures of oxygen and carbon dioxide, pH, and plasma glucose levels) were not affected by the injection of FK506 and were within the reference range.
The whole-blood FK506 concentrations obtained using the PET method correlated significantly with those obtained using the ELISA method (r2 = 0.8126; P < 0.05) (Fig. 4).
Correlation between whole-blood FK506 concentrations obtained by PET and those obtained by ELISA at 5 min (○) and 15 min (•) after injection.
DISCUSSION
In this study, we used 11C-FK506 PET to measure FK506 concentrations in the brain and blood of anesthetized monkeys with PET. The whole-blood concentrations of FK506 found by this method correlated significantly with previously published values obtained using the ELISA method (12). Although in this study we did not examine the metabolite, we confirmed in the preliminary experiment that the 11C-labeled metabolite in whole blood was less than 5% at 15 min after administration of 11C-FK506. This result suggests that the present PET method can measure FK506 concentrations in brain and whole blood as well as does the ELISA method. We measured the concentrations of FK506 after intravenous administration of 0.1 mg/kg—a dose sufficient to show neuroprotective effects in a monkey model of stroke (7). Our results suggest that a brain concentration of 20.0 ng/g for FK506 is sufficient to show efficacy against ischemia in the monkey model of stroke.
In these experiments, brain concentrations of FK506 did not reach a plateau until 7 min after administration and did not increase further, suggesting that FK506 quickly penetrates the blood-brain barrier and remains in the brain for a long time. In a rat experiment, Butcher et al. reported that brain concentrations of FK506 were maintained for more than 72 h after intravenous administration (5). Furthermore, FK506 was also reported to have a long-term neuroprotective effect in focal and global cerebral ischemia in rodents (6). These long-term efficacies presumably are related to the long half-life of FK506 in the brain.
Generally, clinical dose setting has been based on the effective dose or effective blood concentration in animal models. Although the blood level of an agent is easily regulated and confirmed between humans and experimental animals, correlation of brain concentrations—such as might be required for new drugs under development—between humans and experimental animals has not been possible. This may explain, in part, the discrepancy found in the effectiveness of agents between humans and experimental animals. Therefore, as shown in the present study, establishment of a PET method for determining brain concentrations may help improve the accuracy of clinical studies, since PET is the only currently available method for measuring brain concentrations of agents in humans.
CONCLUSION
We succeeded in measuring brain concentrations of FK506 in anesthetized monkeys using 11C-FK506 PET. Our method should be applicable to further PET studies on animal models of stroke and to a human PET study. The present study may also facilitate further applications of the PET technique to new drug development.
Acknowledgments
The authors thank Shigeo Hayashi for operating the cyclotron. The authors also thank Dr. Keizo Yoshida for fruitful discussions, Drs. Kazuo Sakane and Akio Kuroda for synthesizing the 11C-FK506, and Dr. David Barrett for helping with manuscript preparation.
Footnotes
Received Feb. 17, 2004; revision accepted May 17, 2004.
For correspondence or reprints contact: Yoshihiro Murakami, MS, Medical and Pharmacological Research Center Foundation, Wo32, Inoyama, Hakui, Ishikawa, 925-0613, Japan.
E-mail: murakami{at}mprcf.or.jp