Abstract
PET of reporter gene expression holds promise for noninvasive monitoring of gene therapy. Previously, 2 approaches based on the herpes simplex virus type 1 thymidine kinase gene (HSV1-tk) have been successfully applied to the heart. Wild-type HSV1-tk was imaged with 124I-labeled 2′-fluoro-2′-deoxy-5-iodo-1-β-d-arabinofuranosyl-5-iodouracil (FIAU), and a mutant HSV1-tk (HSV1-sr39tk) was imaged with 18F-labeled 9-[4-fluoro-3-(hydroxymethyl)butyl]guanine (FHBG). The aim of this study was to compare these 2 combinations with regard to specificity, imaging contrast, and reporter probe kinetics using dynamic PET in small and large animals. Methods: Similar titers of adenovirus-expressing wild-type HSV1-tk (Adtk), mutant HSV1-sr39tk (Adsr39tk), or control genes were directly injected into the myocardium of 24 rats and 8 pigs. Two days later, dynamic PET was performed with a clinical scanner during the 120 min after injection of 124I-FIAU (Adtk animals and controls) or 18F-FHBG (Adsr39tk animals and controls). Imaging with 13N-ammonia was performed to identify cardiac regions of interest. Results: In rats, significant cardiac 124I-FIAU accumulation occurred in images obtained early (10–30 min) after Adtk injection. Because of tracer washout, however, no difference between Adtk-injected animals and controls was seen in the images obtained later. For 18F-FHBG, specific myocardial accumulation greater than background levels was detected in Adsr39tk-injected animals at early imaging and, in contrast to 124I-FIAU accumulation, increased over time until the latest imaging (105–120 min). At maximum, cardiac 18F-FHBG concentration showed a 4.15 ± 1.65-fold increase compared with controls (105–120 min), and cardiac 124I-FIAU concentration reached a maximal increase of 1.34 ± 0.38-fold compared with controls (10–30 min, P = 0.0014). Global cardiac reporter probe kinetics in rats were confirmed by regional myocardial analysis in pig hearts. Transgene expression was specifically visualized by both approaches. The highest target-to-background ratio of 124I-FIAU in Adtk-infected pig myocardium was 1.50 ± 0.20, versus 2.64 ± 0.49 for 18F-FHBG in Adsr39tk-infected areas (P = 0.01). In vivo results were confirmed by ex vivo counting and autoradiography. Conclusion: Both reporter gene/probe combinations were feasible for noninvasive imaging of cardiac transgene expression in different species. Specific probe kinetics suggest different myocardial handling of pyrimidine (FIAU) and acycloguanosine (FHBG) derivatives. The results favor 18F-FHBG with mutant HSV1-sr39tk because of continuous accumulation over time and higher imaging contrast.
Innovative approaches for cardiovascular molecular therapy are rapidly evolving, and translational efforts from experimental to clinical application are increasing. Gene and cell therapy hold great promise for treatment of heart disease, but despite progress, some basic principles are still under development (1,2). Examples of such open issues are the optimal method for delivery, therapeutic efficacy, the time course and magnitude of gene expression, and the fate of transplanted cells in target and remote areas. The use of reporter genes and radiolabeled reporter probes provides methodology to address these questions noninvasively. Reporter genes can be overexpressed together with therapeutic genes or within transplanted cells, and radionuclide imaging will allow for assessment of the location, magnitude, and persistence of transgene expression in the heart and the whole body (3–6).
Using PET, 2 approaches have been applied for cardiac reporter gene imaging. Both are based on the herpes simplex virus type 1 thymidine kinase gene (HSV1-tk). Using the pyrimidine derivative 124I-labeled 2′-fluoro-2′-deoxy-5-iodo-1-β-d-arabinofuranosyl-5-iodouracil (FIAU) as reporter probe, we recently reported that imaging of wild-type HSV1-tk expression after adenoviral gene transfer in pig hearts is feasible with a clinical PET scanner (6). But in contrast to studies on tumors (7–10), washout of 124I-FIAU from the cardiac area of HSV1-tk expression was found to be a limitation for imaging at later times after injection.
In vivo images were also obtained using the acycloguanosine derivative 18F-labeled 9-[4-fluoro-3-(hydroxymethyl)butyl]guanine (FHBG) and dedicated small-animal PET to identify expression of a mutant herpes HSV1-tk (HSV1-sr39tk) in rats after direct myocardial gene transfer or injection of transfected cells (3,5). 18F-FHBG kinetics in the heart have not been evaluated to date, and potential differences in myocardial uptake and turnover of pyrimidine and acycloguanosine derivatives remain undetermined. The aim of this study was to apply and compare the 2 available reporter gene/reporter probe combinations for cardiac PET in small and large experimental animals.
MATERIALS AND METHODS
Adenoviral Vectors
Replication-defective type 5 adenoviral vectors, which carried either wild-type HSV1-tk (Adtk) or mutant HSV1-sr39tk (Adsr39tk) under transcriptional control of human cytomegalovirus early gene promoter, were constructed and purified as described previously (11,12). Vectors that carried either LacZ gene (AdLacZ) or no exogenous gene (Addl70-3 [AdNull]) were used as negative controls (11). Double cesium chloride–purified vectors were used throughout the study, and titers were determined by plaque assay on 293 cells (13).
Radiolabeled Probes
124I-FIAU was synthesized and purified as previously described (7). With 124I-FIAU, overall radiochemical yield was approximately 70% and radiochemical purity was >98%. 18F-FHBG was synthesized as developed at Forschungszentrum Rossendorf. Briefly, tosylated and methoxytritylated precursor was radiolabeled using K-18F-F/Kryptofix 2.2.2 (Merck) complex, and then protection groups were split off under acidic conditions. The labeling product was purified by high-performance liquid chromatography (HPLC). Radiochemical yield was 5%–15% (decay corrected) after 85–95 min of synthesis. Radiochemical purity was >98%, and average specific activity was 19 GBq/μmol at the end of synthesis.
Rat Protocol
Experimental protocols were approved by the regional governmental commission of animal protection (Regierung von Oberbayern).
Under anesthesia (intramuscular midazolam, medetomidine, and fentanyl), 36 male Wistar rats (268 ± 23 g) received a percutaneous intramyocardial injection into the left ventricular inferior wall from the epigastric angle under echocardiographic guidance (14). Blue dye was injected into 12 animals, which were sacrificed immediately afterward. In short-axis slices (40 μm) of the excised hearts of all animals, the stained area, comprising 14% ± 4% of the left ventricular circumference, was identified, hence demonstrating the reliability and reproducibility of the technique. Subsequently, 2.5 × 109 plaque-forming units (pfu) of either Adtk (n = 7) or AdLacZ (n = 7) in a 200-μL volume were injected, followed by 124I-FIAU PET 2 d later. In another series, 2.5 × 109 pfu of either Adsr39tk (n = 5) or AdLacZ (n = 5) in a 200-μL volume were injected, followed by 18F-FHBG PET 2 d later. After PET, the animals were sacrificed. Their hearts were rapidly removed, weighed, frozen, and analyzed ex vivo.
Pig Protocol
Eight young domestic pigs (20–30 kg) underwent left thoracotomy under anesthesia (isoflurane, 0.4–1.5 vol%; intravenous propofol, 7–10 mL/h; intravenous fentanyl as required). Genes were transferred by regional injection of 1 × 1010 pfu of adenovirus into the anterior myocardium. In 5 animals that were part of a previous study (6), Adtk or AdNull was injected, followed by 124I-FIAU PET 2 d later. In 3 animals, Adsr39tk and AdNull were injected in separate areas of basal and apical anterior wall, followed by 18F-FHBG PET 2 d later. After in vivo PET, the animals were sacrificed. Their hearts were excised, rinsed, and sliced macroscopically into 5 short-axis slices, which were imaged ex vivo.
PET
All PET studies were performed using an ECAT EXACT HR+ clinical scanner (CTI/Siemens). This scanner provides an axial field of view of 15.8 cm, resulting in 47 transverse slices with a slice separation of 3.4 mm. Supine 10-min transmission scans were obtained using 68Ge rod sources. Then, 13N-ammonia images (50–74 MBq for rats, 200–250 MBq for pigs) were obtained for 20 min to assess myocardial localization and perfusion. After a 30-min break to allow for radioactivity decay, a bolus of 124I-FIAU or 18F-FHBG (18–22 MBq for rats, 70–150 MBq for pigs) was injected intravenously, and dynamic imaging (six 10-min frames and four 15-min frames) was performed until 120 min after injection.
Rat Data Analysis.
Emission data, corrected for random events, dead time, and attenuation, were reconstructed using an iterative reconstruction algorithm (ordered-subsets expectation maximization with 4 subsets and 8 iterations). The resulting in-plane resolution of axial images was 5 mm in full width at half maximum (7). Static 13N-ammonia images were used to define circular, 1-cm-diameter regions of interest (ROIs) for the entire left ventricular myocardium on 6–7 transaxial slices. ROIs were copied to identical positions in 124I-FIAU or 18F-FHBG images. Then, the maximal pixels within ROIs in 124I-FIAU or 18F-FHBG images were depicted and maximal cardiac radionuclide concentrations were calculated and expressed as percentage dose per milliliter: (= activity in the maximal pixel within ROIs [MBq/mL]/injected dose [MBq] ×100) (8). Additionally, radioactivity concentrations for liver, thyroid, and stomach were determined.
Pig Data Analysis.
Attenuation-corrected emission images were reconstructed by filtered backprojection. Volumetric sampling was performed to identify 460 myocardial segments in the perfusion study (15). Segments were then transferred to 124I-FIAU or 18F-FHBG images, and polar maps were generated. For regional analysis, a polar map of 9 myocardial areas was applied (anterior, lateral, septal, and inferior walls divided into basal and distal areas; apex as a separate area). Areas of Adtk, Adsr39tk, and AdNull injection, along with remote areas (inferior wall) were defined for each animal according to the experimental protocol. Then, circular ROIs were placed on transaxial images, using 13N-ammonia images for guidance. Regional myocardial concentrations (percentage dose per milliliter) were estimated from maximum pixels within ROIs. The count ratio of 124I-FIAU or 18F-FHBG relative to remote myocardium was calculated for each interval.
Plasma Metabolite Analysis.
In pigs, venous blood was taken at 7, 30, and 120 min for metabolite analysis. Sample collection, preparation, and HPLC analysis for 124I-FIAU were performed as previously described (6). For 18F-FHBG, aliquots (350 μL) were injected onto a Multosphere 100 RP-18 column (5 μm; 150 × 10 mm; flow, 3 mL/min; CS–Chromatographie Service). The gradient was 0%–50% acetonitrile in water containing 0.1% trifluoric acid over 20 min. Detection was performed using a flow scintillation analyzer (Radiomatic 500 TR Series; Packard Canberra Co.).
Ex Vivo Analysis
Rats.
Radioactivity in each heart was measured by γ-counting. Results were expressed as percentage injected dose per gram of tissue. Then, 40-μm slices were prepared (HM500OM microtome; Microm) and digital autoradiography (PhosphorImager 445SI; Molecular Dynamics) was performed for all slices from an animal on the same imaging plate. Ratios of maximal counts in virus injection areas relative to remote area were calculated. LacZ gene expression was assessed by β-galactosidase staining in rats injected with AdLacZ to identify the virus injection site (9).
Pigs.
Macroscopic cardiac short-axis slices were repositioned in the PET scanner. A 10-min transmission scan was obtained, followed by a 30-min emission scan. In attenuation-corrected images, ROIs were defined manually for 9 myocardial areas, similarly to in vivo analysis, and regional tracer concentration was calculated as a percentage of the average of all areas.
Statistical Analysis
Mean values are given with SD. Data were analyzed with JMP 4.0 software (SAS Institute Inc.). Differences were assessed by paired/unpaired t testing as appropriate. Simple linear regression was used to describe relationships between pairs of continuous variables. P values < 0.05 were considered statistically significant.
RESULTS
In Vivo Imaging in Rats
Cardiac Reporter Probe Kinetics.
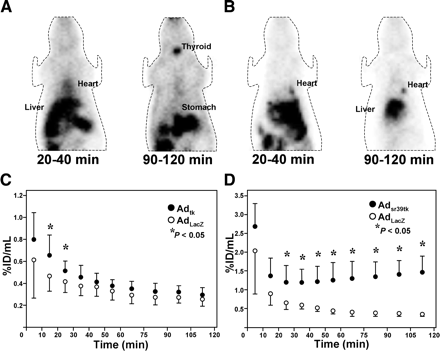
Figure 1 shows examples of whole-body images of reporter probe distribution early and late after injection, along with the time course of myocardial uptake. Significant cardiac 124I-FIAU accumulation was seen in images obtained early after Adtk injection. Because of washout, no difference between Adtk-injected animals and controls was seen at later imaging. For 18F-FHBG, specific myocardial accumulation greater than background levels was detected in Adsr39tk-injected animals at early imaging and, in contrast to 124I-FIAU accumulation, increased over time until the latest imaging. At maximum, cardiac 18F-FHBG concentration showed a 4.15 ± 1.65-fold increase compared with controls (105–120 min), and cardiac 124I-FIAU concentration reached a maximal increase of 1.34 ± 0.38-fold compared with controls (10–30 min; P = 0.0014).
(A and B) PET coronal slices of representative rats at 20–40 min and at 90–120 min after intravenous injection of 124I-FIAU (A) and 18F-FHBG (B), obtained 2 d after intramyocardial injection of Adtk (A) or Adsr39tk (B). (C and D) Cardiac tracer uptake over the entire 2 h after application of radiolabeled probes. Values are mean ± SD in groups after intramyocardial injections with Adtk or negative control (AdLacZ) for 124I-FIAU (C) and with Adsr39tk or AdLacZ for 18F-FHBG (D).
Kinetics in Other Organs.
Kinetics of radioactivity in extracardiac organs were also assessed (Fig. 2). Hepatic 124I-FIAU accumulation was seen in early images but decreased over time. Furthermore, accumulation of radioactivity in thyroid and stomach increased with time in all animals. Differences between Adtk-injected animals and controls were not observed. For 18F-FHBG, hepatic accumulation was pronounced in rats injected with Adsr39tk. A significant increase relative to controls with time after injection was observed.
Kinetics of radiolabeled probes in the liver, 124I-FIAU (A) and 18F-FHBG (B). Values are mean ± SD for each group.
Ex Vivo Validation.
Postmortem analysis revealed a significant correlation between cardiac 124I-FIAU or 18F-FHBG accumulation estimated by PET and γ-counting of the heart (R = 0.789, P = 0.0008 for 124I-FIAU; R = 0.852, P = 0.0017 for 18F-FHBG). Autoradiography demonstrated specific radioactivity accumulation in the regions of Adtk or Adsr39tk injection. The count ratio of 124I-FIAU relative to remote myocardium was 4.03 ± 1.94 in Adtk-injected areas, compared with 1.02 ± 0.30 for AdLacZ areas identified by β-galactosidase staining. The count ratio of 18F-FHBG relative to remote myocardium was 10.08 ± 2.31 in Adsr39tk areas, compared with 1.01 ± 0.11 for AdLacZ areas (P = 0.0001).
In Vivo Imaging in Pigs
Additional experiments were performed on pigs to evaluate interspecies reproducibility. Figure 3 shows representative myocardial images using both approaches and the time course of target-to-background ratios for areas transfected with reporter gene. Coregistered myocardial perfusion was mildly heterogeneous in all animals after myocardial gene transfer, but no perfusion defects were observed.
(A and B) PET slices of myocardial perfusion and early and late reporter probe distribution in pig hearts, 2 d after intramyocardial injection of Adtk (imaged by 124I-FIAU) (A) or Adsr39tk (imaged by 18F-FHBG) (B) into basal anterolateral wall. Shown are representative horizontal long-axis slices (top) and short-axis slices (bottom). (C and D) Values for the count ratio of virus injection area relative to remote myocardium over the entire 2 h after application of radiolabeled probes. Values are mean ± SD for 124I-FIAU (C) and 18F-FHBG (D). LA = left atrium; RA = right atrium; LV = left ventricle; RV = right ventricle.
124I-FIAU/Adtk.
As previously reported, 124I-FIAU uptake was seen to be significantly elevated in all myocardial areas infected with Adtk on images obtained 20–40 min after injection (6). HSV1-tk–expressing areas could no longer be distinguished from blood pool on images obtained 105–120 min after injection. No significant accumulation was observed in AdNull-infected areas or remote myocardium. The count ratio relative to remote myocardium was significantly higher for Adtk-infected areas than for AdNull areas during the first 30 min after injection. The highest count ratio relative to remote myocardium was 1.50 ± 0.20 for 124I-FIAU at 20–30 min.
18F-FHBG/Adsr39tk.
Early images showed 18F-FHBG uptake to be significantly elevated in areas infected with Adsr39tk. In contrast to 124I-FIAU, specific regional uptake continuously increased with time after injection. Starting 30 min after injection, the count ratio relative to remote myocardium was significantly higher than that relative to AdNull areas. The highest target-to-background ratio for 18F-FHBG—2.64 ± 0.49 at 105–120 min—was significantly higher than the highest ratio for 124I-FIAU (P = 0.01).
Plasma Metabolites.
Free iodine was seen for 124I-FIAU and increased with time after injection but remained lower than the peak of labeled 124I-FIAU, as previously described (6). The ratio of free iodine to labeled 124I-FIAU was 0.22 ± 0.18 at 7 min, 0.39 ± 0.33 at 30 min, and 0.56 ± 0.13 at 120 min. Additional metabolites were not identified. For 18F-FHBG, a peak of unidentified radiolabeled metabolite with a shorter retention time was present at HPLC in measurable amounts (about 30%) at 7 min and increased progressively to about 80% of total activity in plasma by 2 h. Activity in the 18F-FHBG peak area divided by that in the total area under the radiochromatogram was 70% ± 1% at 7 min, 23% ± 4% at 30 min, and 19% ± 4% at 120 min. The ratio of unidentified metabolite to 18F-FHBG was 0.44 ± 0.01 at 7 min, 3.4 ± 0.4 at 30 min, and 4.3 ± 0.7 at 120 min. No other peaks were detected, suggesting absence of further metabolites.
Ex Vivo Validation.
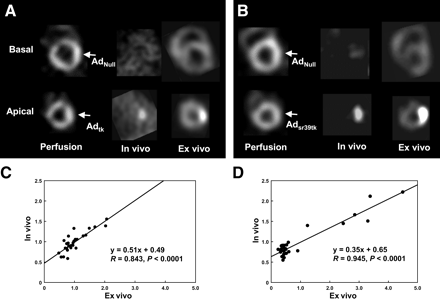
PET of macroscopic heart slices revealed increased radioactivity in areas of Adtk or Adsr39tk injection (Fig. 4). The segmental ex vivo 124I-FIAU concentration correlated significantly with early myocardial in vivo 124I-FIAU uptake (R = 0.843, P < 0.0001), whereas the segmental ex vivo 18F-FHBG concentration correlated significantly with late myocardial in vivo 18F-FHBG uptake (R = 0.945, P < 0.0001).
(A and B) Comparison of in vivo and ex vivo PET images of pig hearts. Shown are short-axis slices of a representative animal that was imaged using 124I-FIAU after intramyocardial injection of AdNull into basal and Adtk into apical anterolateral walls (A) (arrows mark injection site) and of another animal that was imaged using 18F-FHBG after injection of AdNull into basal and Adsr39tk into apical anterolateral walls (B). (C and D) Correlation plots for segmental ex vivo tracer concentration in all animals and early myocardial in vivo 124I-FIAU uptake (C) or late myocardial in vivo 18F-FHBG uptake (D).
DISCUSSION
The usefulness of reporter genes and specific reporter probes for noninvasive imaging is increasingly emphasized because of the potential for in vivo monitoring of the location, magnitude, and persistence of transgene expression in target tissue. There has been considerable experience with nuclear imaging of HSV1-tk reporter gene expression in oncology (7–10,12,16,17). Radioiodinated 124I-FIAU as a pyrimidine nucleoside derivative has preferentially been used for imaging wild-type HSV1-tk expression, whereas radiofluorinated 18F-FHBG as an acycloguanosine derivative has been used for imaging mutant HSV1-sr39tk reporter gene expression. Recently, the usefulness of these techniques has been tested in the heart. A correlation between in vivo reporter probe accumulation and immunohistochemistry of reporter gene product has been demonstrated in both combinations (3–6). To date, several comparisons of these combinations have been performed on tumor cells, but the results conflicted (8,17). Furthermore, transfer of data obtained from tumors to the myocardial setting may be difficult because of different tissue metabolic properties, a different susceptibility for adenoviral infection, and different proliferation rates.
Image Contrast and Reporter Probe Kinetics
We recently demonstrated in another study that infection of myocardial cells with Adsr39tk or Adtk gives rise to comparable amounts of herpesviral thymidine kinase protein messenger RNA. Although the accumulation of 18F-FHBG in vitro was only slightly higher in Adsr39tk-infected cells than in Adtk-infected cells, we did detect considerably less accumulation of 14C-FIAU in mutant TK-expressing cells in this previous project (18). These data supported in vivo use of Adtk as a preferable vector for FIAU imaging and of Adsr39tk as a preferable vector for 18F-FHBG. No further extensive experiments to describe kinetics in vitro were performed in this study, mainly because in vitro observations are difficult to transfer to the in vivo setting. In vivo kinetics are available, and those are of critical importance for practical application.
In vivo kinetics of the 2 reporter probes in this study differed significantly when similar amounts of vector were applied. In HSV1-tk–transfected hearts, specific 124I-FIAU uptake was present only early after injection and decreased subsequently. This observation, which was previously reported for pig hearts (6), was reproducible in rat hearts. At later times after injection, specific tracer uptake was detectable no longer in vivo, but only ex vivo after removal of background activity. In contrast, the kinetics of 18F-FHBG in hearts transfected with mutant HSV1-sr39tk showed a continuous increase of cardiac accumulation with time after injection. In the rats of this study, spatial resolution was limited because of acquisition with a clinical PET scanner. Thus, we also evaluated the myocardial kinetics of 18F-FHBG in a large-animal model, pigs. Probe kinetics were reproducible across species and were associated with higher maximal target-to-background ratios and larger differences between animals expressing reporter gene and control animals.
Myocardial probe kinetics may be influenced by systemic degradation. Metabolite analysis of 124I-FIAU demonstrated deiodination, consistent with previous studies (6,8). Plasma analysis after 18F-FHBG injection in pigs also demonstrated the rapid appearance of a metabolite, which remains unknown but has been previously reported in monkeys, albeit in a smaller amount than we observed in pigs (19). Metabolite analysis of 18F-FHBG has also been reported in humans (20). In our study, the amount of intact labeled reporter probe at the end of imaging was higher for 124I-FIAU than for 18F-FHBG. Thus, systemic degradation cannot explain the observed differences in myocardial kinetics, which have to be attributed to myocardium-specific mechanisms.
In tumors, 124I-FIAU is thought to be taken up by cells, phosphorylated intracellularly by gene product, and then incorporated into host DNA (10). Owing to the lower tissue proliferation rates in myocardium than in tumors, DNA turnover and thus DNA incorporation of nucleotides (phosphorylated nucleosides) is expected to be low. This may facilitate intracellular degradation and tracer efflux. Speculatively, high myocardial expression of nucleotidase enzymes, which are responsible for production of adenosine as a vasoactive and cardioprotective agent, may facilitate intramyocardial dephosphorylation and thus washout of phosphorylated 124I-FIAU (6), and phosphorylated 18F-FHBG may not be a good substrate for these nucleotidase enzymes. To further refine understanding of the apparently complex kinetics of radiolabeled nucleoside reporter probes, detailed studies to dissect the processes of transport, phosphorylation, dephosphorylation, and DNA incorporation will be necessary along with identification and characterization of agents suitable for specific pharmacologic interventions.
Another strength of PET reporter gene imaging is that in addition to heart, other organs can be monitored—for example, to identify vector leakage to systemic circulation as a safety issue for gene therapy. Whole-body imaging of rats allowed for evaluating the feasibility of the 2 probes for this purpose. Gradually increased accumulation of radioactivity in thyroid and stomach after 124I-FIAU injection is related to systemic deiodination and is not specific for reporter gene expression. In the liver as the primary target organ for systemically administered adenovirus in rats (21), the difference in 124I-FIAU uptake between animals receiving intramyocardial injection of ADtk and animals receiving control virus did not reach statistical significance. In contrast, 18F-FHBG liver uptake was pronounced and significantly higher in Adsr39tk-injected rats than in controls. Because systemic vector contamination during intramyocardial injection and egress of adenovirus from the myocardium into systemic circulation are likely to occur in the rat model, 18F-FHBG may be more sensitive than 124I-FIAU for detecting extramyocardial vector leakage.
Practical Implications for Cardiac Reporter Gene Imaging
Although, in comparison with the oncologic setting, the cardiac setting still has only limited experience with in vivo imaging of gene expression, there is great potential for this technique in the monitoring of molecular therapies. Cardiac gene therapy can be evaluated by coexpressing reporter genes and therapeutic genes. Furthermore, reporter genes can be transferred to cells before transplantation for studies of cell trafficking, such as studies of cardiac stem cell therapy (5). The use of heart-specific promoters to drive reporter genes not only may provide tissue-specific readout but also may allow for linkage of the reporter signal to expression of specific endogenous genes in the future (22). This emphasizes the need to identify the most suitable reporter gene and reporter probe combination. Although advantages have been identified for 18F-FHBG and mutant HSV1-sr39tk in this study, reporter genes other than HSV1-tk have been applied in the noncardiac setting. Some, such as dopamine D2 receptor or sodium iodide symporter, have the advantage of producing a gene that lacks immunogenicity (23). Future studies will need to include these alternative approaches for evaluation and comparison to identify an optimal approach for reporter gene imaging of the heart in a given setting.
CONCLUSION
HSV1-tk–based reporter gene and reporter probe combinations are feasible for cardiac transgene expression. Specific reporter probe kinetics suggest different myocardial handling for pyrimidine and acycloguanosine derivatives. Results favor the combination of mutant HSV1-sr39tk reporter gene and 18F-FHBG reporter probe because of continuous accumulation over time and the resultant higher contrast for PET. This approach may therefore be preferable for future monitoring of cardiac gene therapy or cell transplantation.
Acknowledgments
The authors are indebted to Bryan Essien for assistance with vector preparation and to Wolfgang Linke for assistance with preparation of radiolabeled probes. This study was supported by grants from Deutsche Forschungsgemeinschaft (Be 2217/4-1); the Kommission für klinische Forschung, Klinikum rechts der Isar (KKF 8764167); and Fundação de Amparo à Pesquisa do Estado de São Paulo (01/04868-8).
Footnotes
Received Mar. 9, 2004; revision accepted May 20, 2004.
For correspondence or reprints contact: Frank M. Bengel, MD, Nuklearmedizinische Klinik und Poliklinik, Technische Universität München, Klinikum rechts der Isar, Ismaninger Strasse 22, 81675 München, Germany.
E-mail: frank.bengel{at}lrz.tum.de