Abstract
Blockade of lymphocyte recruitment to the intestinal mucosa is considered a useful therapy for inflammatory bowel disease (IBD) and anti-α4 antibodies have clinical benefit in patients with active Crohn’s disease. The aim of this study was to evaluate a scintigraphic technique to assess lymphocyte homing to the colon in 2,4,6-trinitrobenzenesulfonic acid (TNBS)–induced experimental colitis (TNBS colitis) in vivo. Methods: TNBS-sensitized and nonsensitized murine total lymphocytes or CD4+ lymphocytes were radiolabeled with 111In-oxinate. Cells were injected into control mice (n = 5) or mice with TNBS colitis (n = 5). Specific abdominal radioactive uptake was determined by SPECT using a dedicated pinhole system 48 h after cell transfer. Radioactive colon uptake was correlated with histology and colon weight as parameters of inflammation. Results: The radioactive colon uptake was most evident in mice with TNBS colitis that received sensitized lymphocytes (uptake ratio [mean ± SEM], 0.51 ± 0.03 vs. 0.22 ± 0.04; P = 0.004). The sensitized 111In-labeled lymphocytes exacerbated colitis compared with nonsensitized lymphocytes. The colon uptake correlated well with both colon weight and histologic score (R2 = 0.836 and 0.933, respectively). The use of purified 111In labeled CD4+ lymphocytes resulted in a similar scintigraphic pattern. Administration of an anti-α4 antibody decreased radioactivity colon uptake of the 111In-labeled cells compared with the control antibody in mice with TNBS colitis (uptake ratio, 0.72 ± 0.14 to 0.33 ± 0.03; P = 0.012). Conclusion: Animal pinhole SPECT can be applied for temporal and spatial analysis of the lymphocyte homing process in experimental colitis. This technique makes possible the in vivo evaluation of therapeutic efficacy of new drugs that interfere with lymphocyte migration. Moreover, colon uptake of radioactivity can be used as a parameter of disease activity in experimental colitis.
Activated CD4+ T-helper 1 (Th1) lymphocytes, which locally produce proinflammatory cytokines—such as interferon-γ and tumor necrosis factor-α—mediate mucosal inflammation and tissue damage in Crohn’s disease (1). Under normal conditions, naive T lymphocytes migrate randomly from blood to secondary lymphoid tissues for immune surveillance. When naive T lymphocytes encounter an antigen in the draining lymph nodes of the gut, they differentiate into memory or effector cells. If such an encounter takes place in the mesenterial lymph nodes, migration is subsequently directed back to the intestinal mucosa (2). Recruitment of lymphocytes is a multistep process that is controlled by the expression of different sets of adhesion molecules and chemokines (3,4). The integrin α4β7 is the principal gut-homing receptor (5,6). Naive lymphocytes express low levels of α4β7 but, on activation, a significant amount of functionally active α4β7 appears on the surface (7).
Blockade of lymphocyte recruitment to the intestinal mucosa is possibly a useful therapy for inflammatory bowel disease (IBD) (8). Indeed, serial administration of antibodies that bind either the α4 integrin alone or in combination with β7 has resulted in therapeutic benefit in experimental colitis (9,10) and in patients with active Crohn’s disease (11). Hence, interference with mucosal lymphocyte recruitment has become an important goal in the treatment of IBD, but reliable noninvasive methods for serial detection of lymphocyte trafficking in vivo are lacking.
The aim of this study was to design a reliable noninvasive imaging procedure to assess specific recruitment of lymphocytes in experimental colitis. Using SPECT, we first developed a sensitive method to measure the colonic uptake of 111In-oxinate–labeled lymphocytes. The specificity of the technique was subsequently validated using transfer of 2,4,6-trinitrobenzenesulfonic acid (TNBS)–sensitized T lymphocytes in mice with TNBS colitis and blockade of α4-dependent mucosal recruitment.
MATERIALS AND METHODS
All animal experiments were performed with approval and following the guidelines of the Animal Research Ethics Committee of the University of Amsterdam. Female mice (BALB/c, 20–25 g; Charles River) were housed and maintained under standard conditions at our animal care facility. Mice were used at 8–10 wk of age.
For injection and scintigraphy, mice were each sedated by a single intraperitoneal administration of fentanyl/fluanisone (0.375 and 12 μg/g, respectively) (Hypnorm; Janssen Pharmaceutica) and midazolam (6 μg/g; Roche). 111In-Labeled lymphocytes were administered by intravenous injection via the tail vein. The following reagents were used in experiments: CD8α-clone 53-6.7 (gift from Dr. Reina Mebius, Vrije Universiteit Medical Center, Amsterdam, The Netherlands); PS/2, IgG2b, and rat IgG2b (gift from Biogen).
Experiments
The study was divided in 3 experimental protocols to assess lymphocyte migration by SPECT (Table 1). Experiment 1: We first addressed homing characteristics of sensitized and nonsensitized total splenic lymphocytes in TNBS (n = 12) or control mice (n = 10). One mouse died on TNBS induction. Experiment 2: Next, we evaluated whether homing characteristics of sensitized and nonsensitized CD4+ lymphocytes in TNBS (n = 6) or control mice (n = 4) can be imaged with pinhole SPECT. One mouse died on TNBS induction. Experiment 3: Finally, we studied the ability of an anti-α4 integrin antibody to interfere with recruitment of sensitized CD4+ lymphocytes in TNBS colitis (n = 12). Two mice died on TNBS induction.
Visual Uptake and Mean Colon Uptake Ratios of SPECT
Induction of TNBS Colitis.
Colitis was induced by rectal administration of 0.5–2 mg TNBS (Sigma Chemical Co.) dissolved in 40% ethanol (100 μL), using a vinyl catheter positioned 3 cm from the anus, as described (12). Control mice were subjected to an identical procedure, except that 0.9% physiologic saline was the dose. The donor mice, used for lymphocyte isolation from the spleen, received a single dose of saline or TNBS and were sacrificed after 7 d. The recipient mice, used to obtain SPECT images, were administered either saline or TNBS doses (100 μL) on 2 consecutive occasions separated by 7 d (to induce a Th1-mediated delayed-type hypersensitivity response) followed by intravenous injection of labeled donor cells 24 h later.
111In Labeling and Adoptive Transfer.
Lymphocytes from either saline or TNBS mice were used as the source for radioactive labeling and transfer. Briefly, lymphocyte suspensions from the spleen were prepared by squashing the spleens through a 40-μm cell filter strainer (Becton Dickinson) and subsequent red cell lysis with an NH4Cl solution (0.083% NH4Cl, 0.01% KHCO3, and 0.004% ethylenediaminetetraacetic acid, pH 7.4) for 5 min on ice. After a centrifugation step (1,400 rpm; 5 min), the cells were labeled with 111In-oxinate either directly or after CD4+ cell enrichment. For CD4+ cell enrichment, lymphocytes were labeled with the following rat antimouse monoclonal antibodies (mAbs): B220 (clone RA3–6B2), Mac-1 (clone M1/70), and CD8α (clone 53-6.7); the mAb-stained cells were removed in a magnetic field using sheep antirat IgG-coated magnetic beads, according to the manufacturer’s guidelines (Dynal). CD4+ enrichment of total splenic lymphocytes resulted in >60% CD4+ lymphocytes. 111In-Oxinate (1.40 MBq, 25 μL; Mallinckrodt) was added for each 1 × 106 cell pellet and incubated for 15 min at room temperature. Cells were washed once with saline (centrifugation, 400g for 5 min at 20°C; Hettich) and resuspended in saline (200 μL). A cell suspension of the 111In-labeled CD4+ lymphocytes (25 MBq, 200 μL; ∼25 × 106 cells) was injected in randomly assigned TNBS or saline recipient mice on day 7.
Blockade of α4 Integrin.
Rat mAb specific for α4 (PS/2, IgG2b; 1 μg/μL) (13) or an irrelevant isotype-matched control antibody (rat IgG2b; 1 μg/μL) was administered to recipient mice by a single intraperitoneal injection (200 μL) on day 7, after the second dose of TNBS. Similar to the procedure described earlier, the 111In-labeled CD4+ lymphocytes (25 MBq, 200 μL; ∼25 × 106 cells) were treated for 20 min at 4°C with the appropriate antibody (20 μg/mL; 100 μL). Cells were washed once with saline (centrifugation, 400g for 5 min at 20°C) and resuspended in saline (200 μL). A cell suspension of the 111In-labeled CD4+ lymphocytes (25 MBq, 200 μL; ∼25 × 106 cells) was injected in randomly assigned TNBS recipient mice on day 7.
Scintigraphic Imaging
Scintigraphy was performed using recently described and validated high-resolution, pinhole SPECT (14). A γ-camera (ARC3000; Philips) was used with a circular field-of-view diameter of 400 mm. The pinhole collimator has a diameter of 300 mm and an opening angle of 60°. A tungsten pinhole insert of 3-mm aperture was used. All studies were acquired with a 15% energy window on the 173- and 247-keV 111In photo peak. An operating workstation (Hermes; Nuclear Diagnostics) was used to control both the camera and the step motor.
The SPECT acquisition was performed 48 h after intravenous injection of lymphocytes. Dynamic images were acquired at a frame rate of 1 frame per 60 s over 50 min, where projections (64 × 64 matrix) were made in a 360° orbit. SPECT reconstruction was performed using an application program (Hermes; Nuclear Diagnostics) adapted to pinhole SPECT, using filtered backprojection. A Butterworth postreconstruction filter (order, 5; 0.8 cycle/cm) was applied.
Images were interpreted and scored by 2 experienced nuclear medicine physicians. Radioactivity uptake in the colon was scored as follows: no uptake, equal to background; equivocal uptake, higher than background but lower than bone marrow uptake; positive uptake, equal to bone marrow uptake; or manifest uptake, higher than bone marrow uptake.
The colon uptake ratio (CUR) was determined semiquantitatively by regions-of-interest analyses. Five consecutive transverse slices with the highest colon uptake were selected and added. Counts in each region were obtained for the colon, pelvic bone marrow, and abdominal area as background. The CUR was calculated by subtracting background activity from both colon and bone marrow activity and subsequently dividing the corrected colon and bone marrow uptake: (counts colon − counts background)/(counts bone marrow − counts background).
Immediately after SPECT, the mice were sacrificed by cervical dislocation and colons were removed through a midline incision. Isolated colons were cleaned for standard planar pinhole scintigraphy. The colon and blood samples were weighed and counted in a γ-counter (Packard 5530) after SPECT and planar scintigraphy. Results were corrected for decay and expressed as counts per minute (cpm)/gram of tissue. To correct for nonspecific binding, ratios were normalized for radioactivity in blood: (cpm/g colon − cpm/g blood)/(cpm/g blood).
Assessment of Inflammation
The wet weight of the distal 6 cm of the colon was used as an index of disease-related intestinal wall thickening. An experienced pathologist who was unaware of the experimental protocol performed microscopic evaluation on formalin-fixed tissue sections stained with hematoxylin–eosin, using a validated score as described (12). Briefly, because the TNBS-induced ulceration may not be evenly distributed along the colon samples, 2 sections of colons were scored using the following parameters: (a) percentage of colon involved, (b) fibrosis, (c) edema, (d) erosions and ulcerations, (e) crypt loss, (f) infiltration of mononuclear cells, and (g) polymorphonuclear cells. The total score ranged from 0 (normal colon) to a maximum of 23 points (most severe inflammation).
Statistical Analysis
Differences between groups were analyzed by the Mann–Whitney U test. For comparison of multiple datasets, statistical analysis was performed using the Kruskal–Wallis test. Correlation analysis between uptake ratios and histologic scores or colon weights was performed by applying the Spearman ρ-correlation test. Results are expressed as the mean ± SEM. All statistical tests were 2-tailed and differences were evaluated at the 5% level of significance.
RESULTS
Imaging of 111In-Labeled Lymphocytes in Mice with Colitis
Splenic lymphocytes were isolated from both TNBS-sensitized and nonsensitized mice and radioactively labeled with 111In-oxinate, with an efficiency ranging from 60% to 80%. In saline control mice, there was no significant difference in the CUR between sensitized and nonsensitized total lymphocytes at 48 h after injection (Fig. 1). In contrast, uptake of TNBS-sensitized 111In-labeled lymphocytes in mice with established TNBS colitis resulted in a significantly higher CUR than that of nonsensitized lymphocytes (0.51 ± 0.03 vs. 0.22 ± 0.04, P = 0.004; Fig. 1). These observations were confirmed by the images as SPECT slices (Table 1; Fig. 2). These observations indicate that the CUR as determined by SPECT was able to measure specific mucosal lymphocyte recruitment.
Colon radioactivity uptake is increased in mice with TNBS colitis transferred with TNBS-sensitized 111In-labeled lymphocytes. SPECT was performed 48 h after transfer of 111In-labeled TNBS-sensitized or nonsensitized lymphocytes to TNBS and saline control mice. Colon uptake was calculated normalized to bone marrow uptake in pelvis and corrected for background activity: (colon − background)/(bone marrow − background). Box plots of mean radioactivity uptake in 4 groups are shown (donor–acceptor). Overall comparison, P = 0.002; individual comparison between TNBS–TNBS group and 3 other groups, **P = 0.004 (statistically significant).
Increased radioactivity on SPECT of mice with TNBS colitis mice transferred with TNBS-sensitized 111In-labeled lymphocytes. SPECT was performed 48 h after transfer of 111In-labeled TNBS-sensitized or nonsensitized lymphocytes to TNBS and saline control mice. Transverse slices from abdominal region at pelvic level are shown; amount of 111In uptake is color coded from low (black) to high (white). Four images are representative of 4 groups (donor–acceptor). (A) Colon uptake is low in NaCl–NaCl mouse. (B) Colon uptake is equivocal in TNBS–NaCl mouse. (C) Colon uptake is equivocal in NaCl–TNBS mouse. (D) Colon uptake (arrow) is manifest in TNBS–TNBS mouse.
Because the CD4+ cell population is primarily responsible for transferring TNBS colitis (15,16), we analyzed the specificity of 111In-labeled CD4+ spleen cell scintigraphy for migration to both inflamed and healthy colons (Table 1, experiment 2). As expected, there was higher uptake and CUR of sensitized CD4+ lymphocytes versus nonsensitized CD4+ lymphocytes to mice with TNBS colitis. Saline control mice that received sensitized or nonsensitized CD4+ lymphocytes showed low CURs similar to those found after transfer of total lymphocytes (Table 1). The transverse SPECT slices (Table 1) and images of isolated colons confirmed the SPECT results.
Validation of Scintigraphic Colitis Model
Disease activity in the TNBS model was determined as reported (17). The weight of the last 6 cm of the colon was determined on sacrifice on day 9. The colon weight of mice with TNBS colitis that received sensitized lymphocytes (238 ± 24 mg; n = 6) was significantly higher than the weights of mice that received nonsensitized lymphocytes (162 ± 19 mg; n = 5; P = 0.028) and saline mice that received sensitized lymphocytes (114 ± 5 mg; n = 5; P = 0.006) or nonsensitized lymphocytes (101 ± 5 mg; n = 5; P = 0.006; Fig. 3).
TNBS-sensitized lymphocytes exacerbate colitis. TNBS or saline control mice were transferred with TNBS-sensitized or nonsensitized 111In-labeled lymphocytes as indicated (donor–acceptor). Weight of the last 6 cm of colon was determined on sacrifice after SPECT. TNBS–TNBS mice had significantly higher colon weights than weights of mice in other 3 groups. Data represent mean ± SEM. Overall comparison, P = 0.0015; individual comparison between TNBS–TNBS group and 3 other groups, **P < 0.05 (statistically significant).
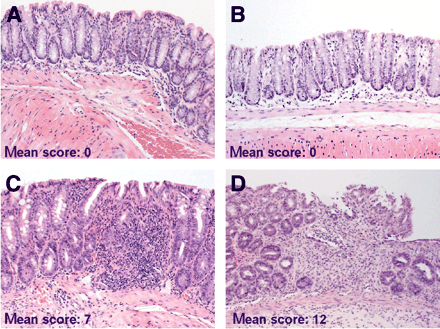
Tissue sections of the colons were examined microscopically to assess whether recruitment of 111In-labeled lymphocytes was associated with histologic signs of inflammatory activity (Fig. 4). Histologic scoring indicated that a higher uptake of sensitized lymphocytes was correlated with higher severity of the induced disease in TNBS colitis compared with nonsensitized lymphocytes (histologic score: 12 ± 0.4 and 7 ± 0.8, n = 5, P = 0.006; Figs. 4C and 4D). In contrast, no pathologic changes were detected in the colons of saline control mice (n = 5; Figs. 4A and 4B). These data suggest that (TNBS-sensitized) 111In-labeled lymphocytes were functional in vivo, based on their accumulation at the diseased site with subsequently increased disease severity.
TNBS-sensitized lymphocytes exacerbate colitis. Extent of mucosal inflammation was graded and mean total histologic scores per group (donor–acceptor) are shown. (A and B) NaCl–NaCl mouse (A) and TNBS–NaCl mouse (B) with normal colon architecture and small number of leukocytes in mucosa. (C) NaCl–TNBS mouse with inflammation (significantly less severe than in D), consisting of edema and influx of inflammatory cells, but no ulcerations and fibrosis. (D) TNBS–TNBS mouse with severe colitis characterized by edema, extensive influx of inflammatory cells, ulcerations, crypt loss, and fibrosis. (Hematoxylin–eosin, ×33)
Mice with TNBS colitis that received sensitized CD4+ lymphocytes had a more severe colitis on histologic evaluation compared with mice that received nonsensitized CD4+ lymphocytes (histologic score, 14 and 10, respectively), although no statistical analysis was performed due to the limited number of mice per group (n = 2). No difference was observed in the histologic score in the healthy control mice that received sensitized or nonsensitized CD4+ lymphocytes (histologic score, 2 and 3, respectively).
SPECT Analysis Versus Disease Severity
To determine whether SPECT could be used to assess disease activity in vivo, we performed a correlation analysis between the CURs and the histologic scores (Fig. 5A) or the colon weights (Fig. 5B). Both the total histologic scores and the colon weights correlated with the CURs in mice with TNBS colitis (R2 = 0.933 and 0.836, respectively; P < 0.001, n = 5).
Colon uptake ratio correlated with parameters of colitis. Mice with TNBS colitis were transferred with TNBS-sensitized or nonsensitized 111In-labeled lymphocytes. Each symbol represents 1 mouse. (A) Significant correlation between histology (x-axis) and CUR (y-axis) (P < 0.001). Correlation coefficient R2 = 0.933. (B) Significant correlation between colon weight (x-axis) and CUR (y-axis) (P < 0.001). Correlation coefficient R2 = 0.836.
Blockade of α4 Integrins Inhibits Lymphocyte Migration into Intestine
Mice with established TNBS colitis (day 9, 48 h after the second TNBS administration) received a single injection with anti-α4 antibody or a control antibody, 4 h before a dose of TNBS-sensitized 111In-labeled CD4+ lymphocytes (experiment 3). Antibody-treated animals compared with control mice showed a lower CUR (from 0.33 ± 0.03 to 0.72 ± 0.14, respectively; P = 0.012, n = 5; Fig. 6A). Visual uptake assessment of the transverse SPECT slices (Table 1) and scanning of the isolated colon samples (Fig. 7) showed the difference between the anti-α4 antibody-treated and the control mice. In addition, γ-counting of the isolated colon corrected for weight and normalized to blood radioactivity showed a decreased radioactivity in the group treated with the anti-α4 antibody compared with the group treated with the control antibody (Fig. 6B; n = 5; P = 0.05).
Colon radioactivity uptake is decreased after anti-α4 antibody injection. SPECT was performed on mice with TNBS colitis 48 h after transfer of 111In-labeled TNBS-sensitized lymphocytes and injection of control antibody or anti-α4 antibody. (A) CUR was calculated normalized to bone marrow uptake in pelvis. Box plots of mean radioactivity uptake of 2 groups are indicated (*P = 0.012 [statistically significant]). (B) Isolated colons were also counted in γ-counter; γ-counter results were corrected for decay and expressed as cpm/g tissue. To correct for nonspecific binding, ratios were calculated normalized to radioactivity in blood: (cpm/g colon − cpm/g blood)/(cpm/g blood) (*P = 0.05 [statistically significant]).
Blockade of CD4+ cell migration to colon after anti-α4 antibody injection. Ex vivo planar scintigraphic images of isolated cleaned colon show distinct colon radioactivity uptake in mouse with TNBS colitis transferred with sensitized 111In-labeled CD4+ lymphocytes (A). Colon radioactivity uptake is decreased after treatment with anti-α4 antibody (B). Images are representative of 5 mice per group.
DISCUSSION
The data presented here show that animal pinhole SPECT is a sensitive method for determining increased lymphocyte recruitment to the inflamed colon in mice. TNBS colitis is characterized by a Th1–cell-mediated transmural colitis and resembles human Crohn’s disease (18). The presence of lymphocytes in the colon was determined visually and semiquantitatively by means of an uptake ratio on transverse slices. The ratio of colon to bone marrow uptake corrects for variables dependent on the exact amount of radioactivity injected and normalizes mice within and between groups. We confirmed the findings of our SPECT analysis with standard planar scintigraphy of isolated colons, γ-counting, and histopathologic parameters of inflammation.
TNBS-sensitized lymphocytes remained functional after 111In labeling. Colon uptake was found to be significantly higher in the inflamed intestine compared with that of the saline control animals, indicating active migration to the intestine. After intravenous injection, lymphocytes enter the circulation via the lungs and then, subsequently, liver and spleen (19). Most nonviable lymphocytes are trapped in the liver (20) and it is believed that unbound radiotracer is not reutilized by lymphocytes but, rather, retained by noncirculating liver macrophages (21–23). It was previously shown that colon uptake increased over time and peaked at 48 h after injection in mice with TNBS colitis (19), suggesting that T lymphocytes are probably involved in the inflammatory response at the colonic ulceration. Finally, accumulation of 111In-labeled TNBS-sensitized lymphocytes might have participated in the inflammatory response with exacerbating signs of intestinal inflammation. Together these data indicate that 111In-labeled lymphocytes homed to the inflamed intestine and that 111In-labeled sensitized lymphocytes participate in the pathogenesis of colitis.
The CUR reflects the severity of inflammation in mice with TNBS colitis. We compared migration of TNBS-sensitized lymphocytes with nonsensitized lymphocytes. The molecular structure of TNBS allows it to bind to autologous colonic proteins, making the complexes immunogenic, presenting via major histocompatibility class II on antigen-presenting cells to Th1 lymphocytes (18). TNBS-sensitized CD4+ lymphocytes acquire an effector/memory phenotype and preferentially migrate to the tissues with high exposure to the inciting antigen (23). In this study, adoptive transfer of total splenic lymphocytes or CD4+ lymphocytes from TNBS-sensitized mice resulted in a significantly higher CUR in the inflamed colon compared with those lymphocytes from nonsensitized mice. Moreover, the mice with TNBS colitis that received sensitized lymphocytes had a more severe colitis as determined by histologic scoring and colon weights. These data are consistent with a study showing that transferred splenic lymphocytes isolated from rats with TNBS colitis migrated to the colon of recipients and exacerbated disease (15). The CUR correlated well with the total histologic score and the colon weight of mice with TNBS colitis. Indeed, the number of lymphocytes in the colon is one of the histologic parameters of colitis severity. These findings prove that the CUR assessed by SPECT can be used as a parameter of disease activity in vivo.
SPECT allows noninvasive temporal and spatial assessment of lymphocyte migration to the inflamed colon. Several techniques have been used for imaging of lymphocyte migration, such as intravital fluorescence microscopy (24,25), bioluminescence assays with luciferase-expressing cells (26), and MRI of T lymphocytes loaded with superparamagnetic nanoparticles (27,28). However, restrictions of these methods include invasiveness, significant modification of donor cells for detection, or analysis of a limited number of anatomic sites or time points. Previous studies have shown that in vivo 2-dimensional planar imaging of radioactively labeled lymphocytes is a technique that allows for in vivo detection of lymphocytes on multiple time points in the entire body (29–31). 111In-Oxinate seems to be the most appropriate radiolabel for in vivo analysis of lymphocyte distribution in animals because of its good cell labeling ability, long half-life (67.2 h), and useful γ-energy peaks (173 and 247 keV) for photon detection (32). Indium-labeled lymphocytes cannot be used in human studies because chromosomal aberrations in these cells have been observed, and possible induction of malignant disease cannot be excluded (33,34). For pinhole SPECT, a minimal amount of radioactivity in the target organ is needed for imaging. With a labeling efficiency of 60%–80% in our study, this resulted in a required mean dose of 1.40 MBq/106 cells, which is considerably higher than the dose used for the in vitro indium labeling and toxicity studies. Detrimental effects of indium isotope labeling, induced by radioionizing effects, chelate toxicity, and chemical toxicity of indium or its decay product cadmium, may affect cell viability (33,35–37). However, as discussed here and elsewhere (19), our results clearly confirm the in vivo functionality of the transferred 111In-labeled lymphocytes.
The specificity of the CUR was first demonstrated by the increased uptake of radioactivity in mice with TNBS colitis that received sensitized lymphocytes and, then, by inhibiting homing of CD4+ lymphocytes to the inflamed intestine (9,18) by blocking the adhesion molecule α4. The inhibition of lymphocyte migration to TNBS colitis in mice was visible from the images after a single dose of an anti-α4 antibody and was confirmed ex vivo by γ-counting.
Based on the increasing knowledge of the molecular basis of leukocyte trafficking, new therapeutic strategies are designed for the purpose of intervening with lymphocyte migration to the inflamed intestine (8). For optimal evaluation of therapy efficacy and understanding of the mechanism of action of new drugs, serial analysis of lymphocyte migration in animal models is a prerequisite for preclinical development. Efficacy of new therapeutic strategies with, for example, chemokine antagonists can be assessed in vivo by this scintigraphic technique.
CONCLUSION
Collectively, the results of this study indicate that SPECT of 111In-labeled lymphocytes is a straightforward and reliable technique to assess lymphocyte migration to the colon in experimental colitis. In addition, the technique provides data on lymphocytes at the cellular level—during the inflammatory response in focal colitis in mice, where the radioactive uptake ratio of the colon can be used as a parameter of disease activity in vivo. Moreover, SPECT will improve the preclinical development of drugs that intervene with lymphocyte migration to the inflamed intestine.
Acknowledgments
We thank Astrid Spijkerboer for technical assistance and Joost Daalhuisen and Ingvild Kop for biotechnical work.
Footnotes
Received Jan. 19, 2004; revision accepted Apr. 12, 2004.
For correspondence contact: Roelof J. Bennink, MD, PhD, Department of Nuclear Medicine, F2-235, Academic Medical Center, P.O. Box 22700, 1100 DE, Amsterdam, The Netherlands.
E-mail: r.bennink{at}amc.uva.nl