Abstract
Bis(N-ethoxy,N-ethyldithiocarbamato)nitrido technetium (V) (99mTc) (99mTcN-NOET) is a new myocardial perfusion imaging agent currently undergoing phase III clinical trials in the United States and in Europe. 99mTcN-NOET cellular uptake has been shown to be inhibited by the calcium channel inhibitor verapamil in cultured newborn rat cardiomyocytes. However, the effect of verapamil on in situ 99mTcN-NOET myocardial uptake remains unknown. Therefore, the aim of this study was to evaluate whether the inhibitory effect of verapamil on the cellular uptake of 99mTcN-NOET shown in vitro could be reproduced in situ in a canine model of normal and ischemic myocardium. Methods: 99mTcN-NOET uptake in normal and ischemic myocardium (70% flow reduction in the left anterior descending coronary artery) was measured in the absence or presence of verapamil (0.015 mg/kg/min × 10 min) in anesthetized, open-chest dogs (n = 17). Control animals were infused with adenosine (0.2 mg/kg/min) to match the verapamil-induced increase in flow. Results: By verapamil treatment, a clinically relevant plasma concentration of the calcium channel inhibitor was attained (mean ± SEM, 290 ± 152 ng/mL). In normal myocardium (n = 8), regional blood flow at the time of 99mTcN-NOET injection was not statistically different in verapamil- and adenosine-treated dogs (1.69 ± 0.03 vs. 1.61 ± 0.04 mL/min/g, respectively). 99mTcN-NOET uptake was slightly higher in the presence of verapamil (0.39 ± 0.01 vs. 0.38 ± 0.01 counts per minute [cpm]/[Bq/kg]/g for adenosine; P = 0.04). However, no significant difference in 99mTcN-NOET myocardial uptake was observed after normalization of the tracer uptake to regional myocardial blood flow. In ischemic myocardium (n = 9), regional blood flow was lower in verapamil-treated than in adenosine-treated animals (0.22 ± 0.02 vs. 0.29 ± 0.03 mL/min/g; P < 0.05). 99mTcN-NOET uptake in the ischemic area was not inhibited by verapamil (0.09 ± 0.01 vs. 0.10 ± 0.01 cpm/[Bq/kg]/g; P = not significant). Conclusion: Verapamil does not inhibit 99mTcN-NOET uptake in situ in normal and ischemic canine myocardium. These results suggest that verapamil should not affect 99mTcN-NOET myocardial uptake in patients referred for myocardial perfusion imaging.
Myocardial perfusion imaging using 201Tl is widely used for diagnostic and prognostic evaluation of patients with suspected or known coronary artery disease. Bis(N-ethoxy,N-ethyldithiocarbamato)nitrido technetium (V) (99mTc) (99mTcN-NOET) is a new myocardial perfusion imaging agent with the better physical characteristics offered by 99mTc (1). As for 201Tl, 99mTcN-NOET uptake (i.e., the amount of 99mTcN-NOET retained in the myocardium after tracer injection and extraction) correlates with regional myocardial blood flow (MBF) over a wide flow range in canine models of coronary artery stenoses associated with pharmacologic stress (2,3). 99mTcN-NOET has also been shown to undergo redistribution in experimental models of transient or sustained myocardial ischemia (2,4). However, unlike 201Tl, the myocardial uptake of 99mTcN-NOET reflected reperfusion MBF, not viability, in an experimental model of reperfused acute myocardial infarction (5). In the clinical setting, no significant difference was found between 99mTcN-NOET and 201Tl with regard to sensitivity and specificity in the detection of coronary stenoses exceeding 50% or 70% (6), and 99mTcN-NOET is currently undergoing phase III clinical trials in the United States and in Europe.
In vitro studies performed using newborn rat cardiomyocytes showed that 99mTcN-NOET cellular uptake is calcium-related because calcium channel inhibitors such as verapamil and diltiazem inhibit the cellular uptake of the tracer by 40% (7). Conversely, calcium channel inhibitors had no effect on 99mTcN-NOET uptake in the isolated rat heart model (8). The possibility that the cellular uptake mechanisms of flow tracers observed in vitro (9,10) could not be seen in vivo has already been demonstrated for 201Tl. Indeed, although ouabain has been shown to inhibit 201Tl uptake in vitro (10), conflicting results have been obtained regarding the effect of this drug on ex vivo (11,12) or in vivo (13,14) 201Tl uptake. On the other hand, dobutamine induced a decrease in 99mTc-sestamibi myocardial uptake in canine models by collapsing the mitochondrial membrane potential (15,16), indicating that the cellular uptake mechanisms shown in vitro for this tracer by Piwnica-Worms et al. (9) could be observed in situ in anesthetized, open-chest dogs.
Accordingly, and considering the frequent use of calcium channel antagonists among patients referred for myocardial perfusion imaging, this study was designed to evaluate whether the inhibitory effect of calcium channel antagonists on the cellular uptake of 99mTcN-NOET shown in vitro (7) could be reproduced in situ in a canine model and, thus, would be of clinical relevance.
MATERIALS AND METHODS
All experiments were reviewed, approved, and performed under the authority of individuals allowed to work on living animals by the French government (C. Ghezzi, authorization 38–01).
Surgical Preparation
Seventeen adult mongrel dogs (mean weight ± SEM, 22.5 ± 1.3 kg) were premedicated with ketamine (10 mg/kg), anesthetized with thiopental (20 mg/kg followed by a maintenance dose of 10 mg/kg/h), and ventilated on a respirator (LKB Medical LB). The left femoral vein was cannulated with an 8-French polyethylene catheter for administration of fluids, medications, and radionuclides. Both femoral arteries were isolated and cannulated with 8-French polyethylene catheters for blood pressure monitoring and microsphere reference blood withdrawal.
A left thoracotomy was performed at the level of the fifth intercostal space, and the heart was suspended in a pericardial cradle. A flare-tipped polyethylene catheter was inserted into the left atrial appendage for microsphere injections. A proximal segment from the left anterior descending coronary artery (LAD) was dissected free of the epicardium and a small-vessel transit time implantable flow probe (model 200–367, Triton Technology Inc.) was placed on the LAD. A snare ligature (2.0 surgical tie) was then placed proximal to the flow probe. The mean arterial pressure (MAP) and heart rate (HR) were monitored (Merit Medical Systems Inc.) and recorded on a personal computer.
Experimental Protocols
Experimental protocols used in this study are depicted in Figure 1. Reagents were obtained from Sigma-Aldrich Co.
Experimental protocol. Verapamil and adenosine doses were 0.015 mg/kg/min × 10 min and 0.2 mg/kg/min, respectively. mic = injection of radioactive microspheres; LAD = left anterior descending coronary artery.
Group 1 (n = 8 Dogs).
After baseline measurements, a first set of radioactive microspheres was injected for determination of baseline regional MBF. Verapamil (0.015 mg/kg/min × 10 min; n = 4 dogs) or adenosine (0.20 mg/kg/min; n = 4 dogs) was then administered intravenously. Fifteen minutes after beginning the verapamil or adenosine treatment, a second set of microspheres was given together with an intravenous bolus injection of 99mTcN-NOET (185 MBq).
Group 2 (n = 9 Dogs).
After baseline measurements and injection of microspheres, the LAD was partially occluded until a 70% reduction in ultrasonic coronary flow was obtained. Five minutes later, a second set of microspheres was injected, and verapamil (0.015 mg/kg/min × 10 min; n = 4 dogs) or adenosine treatment (0.20 mg/kg/min; n = 5 dogs) was begun. 99mTcN-NOET, 201Tl, and a third set of microspheres were simultaneously given 15 min after the beginning of the verapamil or adenosine treatment. 201Tl was given in group 2 dogs because the presence of the LAD stenosis permitted us to obtain a range of regional MBF from the ischemic LAD zone to the normal left circumflex artery (LCx) zone and, thus, allowed the assessment of 99mTcN-NOET’s ability to track flow in the absence or presence of verapamil and to compare it with that of 201Tl.
The dogs were euthanized by an overdose of anesthetic 10 min after 201Tl and 99mTcN-NOET injection.
Determination of Regional MBF Using Radioactive Microspheres and Quantification of Tracer Uptake
The technique used in our laboratory for quantification of regional MBF using radioactive microspheres has been previously described (17). The microspheres (mean diameter, 15 μm; 2–4 million in 3 mL normal saline; Perkin Elmer Life Sciences) were labeled with 51Cr, 103Ru, 95Nb, or 46Sc. The injection order was randomized. Sonication and hand-agitation were performed before their administration into the left atrium over 15 s. Microsphere reference blood withdrawal was begun 10 s before microsphere injection and continued for 2 min after injection. After the dogs were euthanized, the hearts were removed and the left ventricle was sectioned into five 1-cm-thick slices parallel to the base of the left ventricle. Each slice was cut into transmural pieces, which were divided into endocardial and epicardial segments of roughly 1 g each. Approximately 80 myocardial segments were obtained for each dog. The myocardial segments and arterial blood samples were counted for 201Tl or 99mTc activities in a γ-well scintillation counter (Cobra II; Packard Instruments) within 24 h using window settings of 50–100 keV and 120–180 keV, respectively.
Four days later, when 99mTc activity was negligible, the myocardial samples were counted for microsphere regional MBF determination. The microsphere window settings were 51Cr, 230–330 keV; 103Ru, 450–550 keV; 95Nb, 640–840 keV; and 46Sc, 730–900 keV. All tissue counts were corrected for background, isotope spillover, and decay during the time of counting, and regional MBF was calculated using PCGERDA software (Scientific Computing Solutions, LLC). Injected doses of 99mTcN-NOET and 201Tl were calculated after assessment of the activity present in the syringe before and after intravenous administration using a dose calibrator (CRC-15R; Capintec) and were normalized to the animal’s weight and expressed as Bq/kg. Tracer tissue activities (counts per minute [cpm]) at the time of injection were calculated after appropriate decay correction, and tracer uptakes were normalized to the injected dose and the sample weight (g). 99mTcN-NOET and 201Tl myocardial uptakes were then expressed as cpm/[Bq/kg]/g (raw uptake) and were also subsequently normalized to MBF (in mL/min/g) in each segment (normalized uptake). In group 1, all myocardial segments were pooled. In group 2, segments with stenosis flow ≤50% of baseline flow were considered ischemic. In this group, mean myocardial activities of 201Tl and 99mTcN-NOET (cpm/[Bq/kg]/g) and MBF were also expressed as a percentage of the mean activity and MBF from the normal, nonischemic zone from each individual animal.
Determination of Plasma Verapamil Concentration
In group 1, a 1.5-mL arterial blood sample was withdrawn before 99mTcN-NOET injection (15 min after intravenous verapamil) and centrifuged (1,900 rpm × 2 min at 4°C). One-half milliliter of plasma was used for determination of verapamil concentration. Plasma proteins were precipitated by adding 1 mL of a 2.5% H2SO4/97.5% isopropanol solution. After centrifugation, 0.1 mL of 10N H2SO4 and 1 mL of heptane were added to the supernatant. After shaking and centrifugation (1,000 rpm × 2 min at room temperature), 1 mL of the inferior aqueous phase was neutralized with 0.9 mL of 1N NaOH. Fifty microliters of this solution were then analyzed by high-performance liquid chromatography (HPLC) using a C18 column and a 65% 0.1 mmol/L phosphate buffer/35% acetonitrile (pH 7.4) solution. The flow rate was 0.7 mL/min and absorbance was read at 203 nm. Under these conditions, complete elution of plasma verapamil occurred after 21 min. Extraction efficiency was determined by adding known amounts of verapamil to whole blood samples, and standards were also performed for calibration purposes.
Statistical Analysis
Results are expressed as mean ± SEM. Statistical analyses were performed using SYSTAT software (SYSTAT Software, Inc.). Within-group and between-group analyses were performed using repeated measures ANOVA or 1-way ANOVA, respectively. Upon identification of significance by repeated measures ANOVA, within-group post hoc testing was performed using Bonferroni tests. A P value of <0.05 was considered statistically significant.
RESULTS
Group 1
Hemodynamics.
Hemodynamic parameters for group 1 are presented in Table 1. Adenosine (0.2 mg/kg/min) and verapamil (0.015 mg/kg/min × 10 min) induced a 23% and 12% decrease in MAP versus baseline at the time of 99mTcN-NOET injection, respectively (P = not significant). HR was not affected by the presence of adenosine or verapamil. HPLC assays indicated that the 0.015 mg/kg/min × 10 min infusion of verapamil resulted in a plasma concentration of 290 ± 152 ng/mL at the time of 99mTcN-NOET injection.
Hemodynamic Parameters
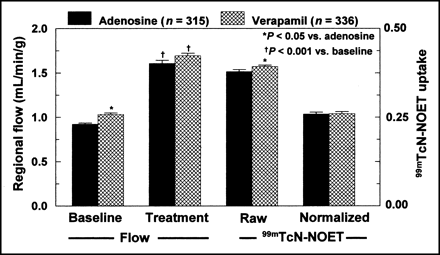
Regional MBF and 99mTcN-NOET Uptake.
Results are presented in Figure 2. Baseline flow was slightly lower in adenosine-treated dogs than in verapamil-treated dogs (0.92 ± 0.01 vs. 1.03 ± 0.02 mL/min/g, respectively; P < 0.05). Adenosine and verapamil infusion induced a 74% and a 64% increase in flow, respectively (P < 0.001 vs. baseline). At the time of 99mTcN-NOET injection, regional MBFs in adenosine- and verapamil-treated dogs were not significantly different (1.61 ± 0.04 vs. 1.69 ± 0.03 mL/min/g, respectively; P = not significant). 99mTcN-NOET myocardial uptake was not inhibited by the presence of circulating verapamil. A 3.8% increase in tracer uptake from the adenosine-treated to the verapamil-treated dogs was observed (0.38 ± 0.01 vs. 0.39 ± 0.01 cpm/[Bq/kg]/g; P = 0.04), which occurred in the presence of a 5.3% higher regional MBF in adenosine- versus verapamil-treated animals (P = 0.06). After normalization of tracer uptake to MBF, no significant difference was observed in 99mTcN-NOET myocardial activity between adenosine- and verapamil-treated animals.
Regional MBF and 99mTcN-NOET myocardial uptake in group 1 dogs with either adenosine or verapamil treatment. Raw and normalized 99mTcN-NOET uptake is expressed as cpm/[Bq/kg]/g and as cpm/[Bq/kg]/g normalized to MBF (in mL/min/g), respectively.
Group 2
Hemodynamics.
Hemodynamic parameters for group 2 are presented in Table 1. LAD stenosis induced a 35% (P < 0.05 vs. baseline) and a 14% (P = not significant vs. baseline) decrease in MAP in the adenosine- and verapamil-treated dogs, respectively. Adenosine treatment did not affect MAP, whereas verapamil further decreased MAP by 25% (P < 0.05 vs. ischemia). At the time of 99mTcN-NOET and 201Tl injections, MAP was not significantly different in the adenosine- and verapamil-treated dogs. HR was not affected by LAD stenosis or adenosine, whereas verapamil decreased HR by 23% (P < 0.05 vs. ischemia).
Regional MBF and 99mTcN-NOET Uptake.
Results are presented in Figures 3A and 3B. In the normal zone, baseline flows were not statistically different in the adenosine- and verapamil-treated dogs (1.02 ± 0.05 vs. 0.98 ± 0.04 mL/min/g, respectively; P = not significant). Flow decreased in the adenosine-treated dogs after the stenosis was set (0.85 ± 0.04 mL/min/g; P < 0.05 vs. baseline) and remained unchanged in the verapamil-treated dogs (0.96 ± 0.07 mL/min/g; P = not significant vs. baseline). Adenosine and verapamil had no effect on regional MBF in group 2 dogs. Myocardial flows in the normal zone of adenosine- and verapamil-treated dogs were not statistically different at the time of tracer injection. There was no statistical difference in normal zone 99mTcN-NOET uptake between adenosine- and verapamil-treated animals (0.28 ± 0.01 vs. 0.30 ± 0.01 cpm/[Bq/kg]/g, respectively; P = not significant) (Fig. 3A).
Regional MBF and 99mTcN-NOET myocardial uptake in remote (A) and ischemic (B) zones of group 2 dogs with either adenosine or verapamil injections. Raw and normalized 99mTcN-NOET uptake is expressed as cpm/[Bq/kg]/g and as cpm/[Bq/kg]/g normalized to MBF (in mL/min/g), respectively.
In the ischemic zone, baseline flows were not statistically different in the adenosine- and verapamil-treated dogs (0.90 ± 0.05 vs. 0.92 ± 0.06 mL/min/g, respectively). After the LAD stenosis was set, flow decreased by 70% in both subsets of dogs (0.27 ± 0.02 and 0.29 ± 0.03 mL/min/g for adenosine and verapamil, respectively; P < 0.001 vs. baseline). Flow remained unchanged during adenosine infusion (0.29 ± 0.03 mL/min/g) but further decreased after verapamil treatment (0.22 ± 0.02 mL/min/g; P < 0.05 vs. ischemia). Myocardial flow in the ischemic zone at the time of tracer injection was slightly higher in the adenosine-treated than in the verapamil-treated dogs (P < 0.05). 99mTcN-NOET uptake in the ischemic zone was not statistically different between adenosine- and verapamil-treated animals (0.10 ± 0.01 vs. 0.09 ± 0.01 cpm/[Bq/kg]/g, respectively; P = not significant). There was no significant difference in tracer uptake between adenosine- and verapamil-treated animals after normalization to MBF.
Correlation Between 99mTcN-NOET and 201Tl Myocardial Uptake with MBF in Presence of Coronary Stenosis.
Results are presented in Figure 4. 99mTcN-NOET and 201Tl uptakes were significantly correlated with MBF in the adenosine group (r = 0.98 and 0.97, respectively; P < 0.001). This linear relationship did not differ in the verapamil-treated dogs (r = 0.98 and 0.96 for 99mTcN-NOET and 201Tl, respectively; P < 0.001).
99mTcN-NOET (A) and 201Tl uptake (B) plotted as function of MBF in myocardial segments from group 2 dogs with either adenosine or verapamil injection. Mean myocardial activities of 201Tl and 99mTcN-NOET and MBF are expressed as percentage of mean tracer activities and MBF from normal, nonischemic zone from each individual animal.
DISCUSSION
99mTcN-NOET is a new perfusion imaging agent. Its cellular uptake has been shown in vitro to be calcium related because calcium channel inhibitors such as verapamil and diltiazem inhibited its uptake by 40% in newborn rat cardiomyocytes (7). Considering the widespread clinical use of calcium antagonists, this study was undertaken to assess the effect of the calcium channel inhibitor verapamil on 99mTcN-NOET myocardial uptake in situ in a canine model.
The 0.015 mg/kg/min × 10 min infusion of verapamil resulted in a plasma concentration of the drug of 290 ± 152 ng/mL, comparable with the 120 ± 20 ng/mL plasma concentration reported in chronically treated patients (18). Thus, the dose used in this study permitted the plasma concentration of the calcium antagonist to reach the same range as observed clinically.
In animals not subjected to coronary flow reduction (group 1), the decrease in MAP after verapamil injection was comparable with that observed by Pagel et al. (19) after a verapamil intravenous bolus injection of 0.1 or 0.2 mg/kg in dogs with no coronary stenosis, and the verapamil-induced increase in regional MBF was similar to that reported by Neugebauer with the same inhibitor dose (20). Control animals were infused with adenosine to match the flow increase produced by verapamil. Adenosine has been shown to have no effect on 99mTcN-NOET myocardial extraction (3). No inhibition of 99mTcN-NOET myocardial uptake was observed in the presence of verapamil. The 3.8% higher 99mTcN-NOET myocardial uptake in verapamil-treated dogs (P = 0.04 vs. adenosine-treated dogs) occurred in the presence of a 5.3% difference in flow between the 2 groups at the time of 99mTcN-NOET injection (P = 0.06). The absence of effect of the calcium antagonist was confirmed by the fact that normalization of 99mTcN-NOET uptake to MBF removed the observed differences in raw uptake.
Animals from group 2 were subjected to a 70% LAD flow reduction to evaluate the effect of verapamil on 99mTcN-NOET uptake in ischemic myocardium. The decrease in MAP after placement of the stenosis was similar to that reported by Thuillez et al. after a 60%–75% reduction in LCx blood flow (21). Normal zone flow was significantly decreased in adenosine-treated dogs as a result of a greater decrease in MAP. However, normal zone flow of both adenosine- and verapamil-treated dogs remained in the range of normal myocardium (0.8–1.2 mL/min/g). Regional MBF was significantly decreased by 70% in the ischemic LAD zone of both subsets of dogs.
Myocardial flow in the normal zone was unaffected by verapamil, in accordance with the results described by Urquhart et al. for the same inhibitor dose in a canine model of subtotal coronary occlusion (22), whereas flow was significantly decreased in the ischemic zone after the administration of the drug, as described by Thuillez et al. (21). These effects of verapamil on regional MBF could be explained by the fact that measurements were made early (15 min) after drug administration—that is, at a stage when the verapamil-induced decrease in MAP was maximal. It has been shown that the maximal decrease in MAP and the resulting drop in coronary perfusion pressure induced by calcium channel antagonists is concomitant with early negative inotropic effects and might lead to a transient decrease in flow in the ischemic zone as well as to an impairment of the verapamil vasodilator effect in the normal zone (21). The greater drop in pressure after LAD stenosis in the adenosine-treated animals could explain the failure of adenosine to increase MBF (23). Finally, at the time when the tracers were injected, regional MBF in the normal zone was not statistically different between adenosine- and verapamil-treated dogs and was decreased by 24% in the ischemic zone of the verapamil-treated dogs compared with adenosine-treated animals. 99mTcN-NOET myocardial uptake was normalized to MBF to account for flow differences between adenosine- and verapamil-treated animals at the time of tracer injection and was not inhibited by the presence of verapamil in the normal and ischemic zones. As depicted in Figure 4, 99mTcN-NOET myocardial uptake, similar to that of 201Tl, is strongly correlated with MBF in dogs treated with either adenosine or verapamil, providing further evidence that calcium channel inhibitors are not inhibiting 99mTcN-NOET uptake in vivo in our canine model.
99mTcN-NOET cellular uptake mechanisms are related to calcium channels because calcium channel antagonists such as verapamil and diltiazem inhibited its uptake by 40% in newborn rat cardiomyocytes (7). However, this effect of some calcium channel inhibitors on 99mTcN-NOET myocardial uptake was not observed in the isolated perfused rat heart model (8). A subcellular distribution study suggested that 99mTcN-NOET cellular uptake occurs on plasma membranes (24). Furthermore, studies performed on isolated rat hearts using a detergent such as Triton X-100 or perfusion with cyanide (25) or red blood cells (26) indicated that 99mTcN-NOET myocardial kinetics were immediately sensitive to the composition of the perfusing buffer, leading to the hypothesis of an endothelial localization of the tracer. Taken together, these ex vivo studies suggest that fixation of 99mTcN-NOET occurs on plasma membranes of endothelial cells on this whole organ model. Because endothelial cells do not express voltage-dependent calcium channels (27), this may explain the lack of effect of calcium channel inhibitors on 99mTcN-NOET myocardial uptake observed ex vivo in the isolated rat heart model (8) and in situ in this study. Our study demonstrates that the in vitro cellular uptake mechanisms of 99mTcN-NOET could not be observed in situ on anesthetized, open-chest dogs.
There are several limitations in this experimental study. First, the thiobarbiturate thiopental used for anesthesia has been shown to affect ionic currents. The effect of this drug on voltage-dependent calcium channels is controversial (28,29). However, although the possibility exists that thiopental could have affected 99mTcN-NOET myocardial uptake, this potential effect related to anesthesia would have been present in all experimental groups and should not have masked an effect of verapamil. Second, an alternative explanation for the discrepancy in results obtained in our previous in vitro study (7) and in this in situ study on anesthetized dogs is that the dose of verapamil used on cultured cardiomyocytes abolished cellular contractility, whereas contraction was maintained in this study. Therefore, the proportion of blocked calcium channels in situ in anesthetized dogs was lower than that in vitro and may not have been sufficient to inhibit the myocardial uptake of 99mTcN-NOET. Finally, another problem related to the dose of verapamil is that the inhibitor had little or no effect on MAP and HR in group 1 dogs. Although the plasma concentration of verapamil was found to be in the clinical range, there is a possibility that the effective concentration of the drug in canine plasma is higher than that in human plasma. However, verapamil increased MBF by 64%, suggesting that the dose used in our study was effective.
CONCLUSION
Unlike our previous in vitro observation showing inhibition of 99mTcN-NOET cellular uptake by calcium channel inhibitors such as verapamil and diltiazem, we found that verapamil does not inhibit 99mTcN-NOET myocardial uptake in normal or ischemic canine myocardium in situ. These data suggest that verapamil should not affect 99mTcN-NOET myocardial uptake in patients referred for myocardial perfusion imaging and, therefore, should not alter its diagnostic accuracy for the detection of coronary artery disease.
Acknowledgments
This experimental study was supported by research grants from Cis Bio International–Schering S.A., Gif-sur-Yvette, France, the Institut National de la Santé et de la Recherche Médicale, France, and the Ministère de l’Education Nationale, de la Recherche et de la Technologie, France. The authors have no conflict of interest and no financial relationship to disclose.
Footnotes
Received Aug. 28, 2002; revision accepted Feb. 12, 2003.
For correspondence or reprints contact: Daniel Fagret, MD, PhD, Laboratoire d’Etude de Radiopharmaceutiques, INSERM E0340, Faculté de Médecine de Grenoble, Domaine de la Merci, F-38700 La Tronche, France.
E-mail: DFagret{at}chu-grenoble.fr