Abstract
Our goal was to study cerebral blood flow (CBF) changes after surgery in a group of 15 patients with idiopathic normal pressure hydrocephalus (NPH). Methods: We used hexamethylpropyleneamine oxime SPECT and statistical parametric mapping (SPM), an image analysis method that does not require prior selection of regions of interest. Results: Our study showed areas of significant increase in perfusion in specific regions of both frontal lobes and the right parietal lobe. Regions of increased perfusion were found in the left prefrontal dorsolateral areas (Brodmann’s areas 9 and 45 or 47), right frontal premotor area (Brodmann’s area 44), right medial prefrontal region (Brodmann’s area 10 or 32), right frontal white matter area (superior longitudinalis fasciculus), and right basal ganglia (lenticular nucleus, putamen, and globus pallidus). In the right hemisphere, another region of increased perfusion was found in the inferior parietal lobule (Brodmann’s area 40). The 2 areas most related to clinical improvement were Brodmann’s area 32 and the frontal part of the left lobule of Reil insula. Conclusion: The results obtained with the SPM method of image analysis confirm and expand on previous CBF literature in NPH, with specific CBF regions located in frontal and parietal areas that improve after surgery in idiopathic NPH.
Cerebral blood flow (CBF) in normal pressure hydrocephalus (NPH) has been studied since soon after the description of the syndrome by Adams et al. in 1965 (1). Much of the research has focused on the need to find a reliable diagnostic procedure to predict which patients will benefit from shunting and to obtain a better understanding of the pathophysiology of the condition. Preoperative studies have reported a reduction in CBF in patients with NPH (2–8). Some authors have found a diffusely decreased CBF (2–4,9). Other studies have reported focal abnormalities, but without any specific pattern in their localization (2,10,11), and still others have demonstrated cerebral regional decreases that mainly affect anterior areas (5,6,12–16).
After surgery, improvement or restoration of CBF has been observed, mainly in the frontal lobes (6,12,14). However, improved CBF has also been reported after shunt surgery in other cerebral regions, such as the temporal, parietal, and occipital cortex, central subcortical region, basal ganglia, hippocampus, and mesencephalon (6,12,14,17).
These contradictory findings are probably due to the diversity in the methodology used for CBF acquisition and analyses. Many of the studies used nontomographic procedures. Furthermore, the methods used for CBF analysis are in most cases semiquantitative or visual; they include only a few slices, and consider gross preestablished regions of interest. Our aim was to study CBF changes in a group of patients with idiopathic NPH using hexamethylpropyleneamine oxime (HMPAO) SPECT and Statistical Parametric Mapping (SPM), a software package that offers an objective, quantitative voxel-by-voxel analysis and that has become accepted as a standard approach in the analysis of brain activation studies (18). This procedure has the advantage of working without any a priori anatomic assumption regarding the nature of the changes and has been considered a powerful tool for the analysis of SPECT data (19).
MATERIALS AND METHODS
Subjects
Subjects included in the study were 31 consecutive patients studied for NPH in the Neurosurgery Department of Vall d’Hebron University Hospital from January 1998 to September 2000. One patient could not receive surgery for medically unrelated reasons and another patient did not undergo shunting at the request of his family. One patient died of a cerebrovascular accident before the control assessment, 8 patients did not perform the postsurgical SPECT analysis, another patient had a secondary hydrocephalus, and the SPECT results in 4 patients were discarded because strong facial increased uptake meant that satisfactory analysis with SPM could not be performed. Therefore, the analysis covered 15 patients with idiopathic NPH and satisfactory CBF measurements performed before and 6 mo after shunting (mean ± SD, 6.5 ± 2.0 mo). All patients were free of any type of medication with a potential influence on cerebral perfusion, such as nimodipine or citicoline, for at least 5 d before the first SPECT analysis. No medication of this kind was administered afterward. Two patients had achondroplasia and hydrocephalus that presented during adulthood as NPH. Informed consent for all aspects of the study was obtained from each patient or from a relative.
The patients included 9 men and 6 women (age, 56–81 y; mean ± SD, 73.5 ± 7.4 y). All patients presented ventricular dilatation (Evans index ≥ 0.30) and a history of gait disturbances, dementia, or sphincter dysfunction. In accordance with our protocol for study and management of NPH (20–22), the decision to insert a shunt in a patient was based on clinical, neuroimaging (CT or MRI), continuous intracranial pressure monitoring, and cerebrospinal fluid (CSF) dynamic studies. The coexistence of cerebrovascular disease or other neurodegenerative disorder is not an exclusion criterion for shunting in our institution. Due to the age range of the sample, many of the patients had cerebrovascular risk factors (indeed, only 3 [20%] had none).
Continuous intracranial pressure (ICP) was monitored using a fiber optic extradural device (LADD Research Industries, Inc.) for at least 48 h, including overnight recording. We evaluated the presence of A waves (ICP elevations at least 20 mm Hg above the resting line, with abrupt onset and end, and lasting between 5 and 20 min) and B waves (0.5–2 ICP waves per minute, lasting for at least 10 min) and calculated their percentage during the total monitoring time. In all patients, the diagnosis of an active (mean ICP > 12 mm Hg) or compensated (mean ICP ≤ 12 mm Hg but with the presence of A or B waves) hydrocephalus was confirmed, for which the therapeutic intervention was judged to be appropriate. CSF dynamics (resistance to outflow and conductance to outflow) were determined by Katzman and Hussey’s constant rate infusion test (23). Table 1 displays clinical summaries of the patients.
Demographic and Clinical Characteristics of Patients Before and After Surgery
Surgical Method
All patients underwent ventriculoperitoneal shunting. The ventricular catheter was always placed in the right frontal horn. A Hakim Programmable Valve (Medos S.A., Johnson and Johnson Co.) was implanted in 1 patient; a Hakim Precision Valve 40 + 10 mm H2O (Medos S.A., Johnson and Johnson Co.) was used in 7 patients; a Delta Valve, performance level 0.5 (Medtronic PS Medical), was used in 2 patients; a gravity-compensating accessory (GCA) (NMT Neurosciences Implants S.A.) was used in 2 patients; a low-pressure Novus Valve (Heyer-Schulte NeuroCare, L.P.) was used in 2 patients; and a median-pressure Novus Valve (Heyer-Schulte NeuroCare, L.P.) was used in 1 patient.
All patients underwent successful shunt surgery. One patient suffered an asymptomatic subdural collection of no clinical importance, which resolved spontaneously. The rest of the patients did not present any surgical complications either at 1- or 6-mo follow-up.
Measures of Surgical Outcome
The clinical status and the capacity for daily life activities of all patients were assessed before surgery and 6 mo after the shunting procedure. We used the NPH scale (20), which evaluates the 3 main symptoms of the disease and ranges from 3 (unable to deambulate, severe dementia, and double incontinence) to 15 (normal gait, cognition, and sphincter function); a modification of the Stein and Langfitt scale (24), which includes 5 grades: from 0 if there is no neurologic deficit and the patient is able to work or perform the same duties as before the disease to V if the patient is bedridden or vegetative; and the Informant’s test, which registers the functional behavioral changes and consists of 17 items scored on a 5-point basis: 1 = much better, 2 = slightly better, 3 = no change, 4 = slightly worse, and 5 = much worse.
SPECT Image Acquisition and Analysis
SPECT conditions were standard: insertion of the venous line and a sensorial resting period of 20 min before administration of the dose. Brain SPECT was performed 10–20 min after intravenous injection of 800 MBq 99mTc-HMPAO (Ceretec; Amersham Health) using a rotating, dual-head γ-camera (Helix HR; Elscint-General Electric) equipped with high-resolution, parallel-hole collimators. Data were acquired in a 128 × 128 matrix through 360° rotation at 3° intervals for 25 s per view. The pixel size acquired was 0.22 cm. The average radius of rotation was 16 cm. Approximately 5–6 million counts were acquired per patient. Data reconstruction of transaxial slices was performed by filtered backprojection (Metz filter power, 3.00; full width at half maximum, 10 mm) and subsequent attenuation correction using the Chang method (attenuation coefficient, 0.12). The reconstruction pixel size was the same as the one acquired.
The SPECT images were analyzed using the SPM99 image analysis software (Wellcome, Department of Cognitive Neurology, Institute of Neurology, University College London, London, U.K.). All images were manually reoriented using the SPM software to make them coincide as closely as possible in origin and orientation with the SPECT template image of the SPM. In 4 of the 15 selected patients, there was a slight facial contamination of the image. A mask created with MRICRO software (developed by Chris Rorden; University of Nottingham Psychology; Nottingham, U.K.) was used to filter the extracerebral signal. The spatial transformation of all SPECT images into the Talairach coordinate system was performed using the SPM’s spatial normalization function. This function was used with the sinc interpolation (9 × 9 × 9) to transform the functional space into a standard space for all subjects, the MNI space (Montreal Neurological Institute), and used the reference SPECT image provided by the SPM software, which had already been transformed into the Tailarach space. The normalized SPECT images were then smoothed using a 3-dimensional Kernel gaussian filter of 8 × 8 × 8 mm.
We performed a multisubject analysis with 2 conditions (before and after surgery) and 0 covariates. Only voxels with intensities > 80% of the global normalized image mean were included in the statistical analysis (mean value calculated automatically). The critical threshold for this analysis was set at a score corresponding to a P value < 0.05 corrected for multiple comparisons. Only significant clusters ≥ 20 voxels (activated regions > 8 mm2) were selected and considered significant areas of increased perfusion. Significant CBF changes were displayed in SPM as statistical parametric maps in the Talairach space both as the probabilities of cluster size and the activation magnitudes based on Z scores.
We performed a regression analysis to examine whether there was a relationship between clinical improvement and CBF changes. We used the simple regression SPM99 analysis between the SPECT images for each subject (and condition) and the NPH scores. The significant threshold was P corrected < 0.05.
RESULTS
Clinical Status and Outcome
At presurgical assessment, 12 patients presented the complete clinical triad, and 3 patients showed gait and cognitive dysfunction with normal sphincter control. According to the NPH scale, 14 patients (93.3%) improved 1 or more grades on the total score at 6-mo follow-up. Twelve patients (80%) showed gait improvement, 7 patients (46.7%) showed some degree of cognitive amelioration, and in 11 patients (91.7%) sphincter control was also improved (Fig. 1).
Postshunt improvement in gait, cognition, and sphincter functioning according to NPH scale.
Regarding daily life activities, before surgery, 4 patients (26.7%) were independent for daily life activities (functional grades 0, I, and II of Stein and Langfitt scale), 2 patients (13.3%) required some help or supervision (grade III), and 9 patients (60%) were totally dependent (grades IV and V). Six months after shunt surgery, 9 patients (60%) were able to cope independently with daily life activities, 3 patients (20%) were partially dependent, and the remaining 3 patients (20%) remained dependent.
Comparison between presurgical and postsurgical clinical and functional scales using the Wilcoxon matched-pairs signed rank test yielded statistically significant improvements in all measures: NPH scale (Z = −3.27; P = 0.001), NPH gait (Z = −2.97; P = 0.003), NPH cognitive (Z = −2.37; P = 0.02), NPH sphincter control (Z = −2.93; P = 0.003), Stein and Langfitt scale (Z = −2.55; P = 0.01), and Informant’s test (Z = −2.18; P = 0.03).
CBF Results
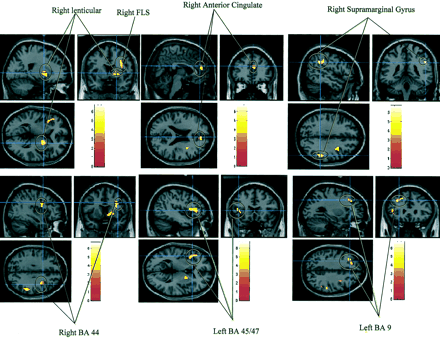
As can be seen in Table 2 and Figure 2, comparison between presurgical and postsurgical SPECT images of the 15 patients using the SPM showed significant areas of increased perfusion in the left prefrontal dorsolateral areas (Brodmann’s areas 9 and 45 or 47), a right frontal premotor area (Brodmann’s area 44), and a right frontal white matter area (superior longitudinalis fasciculus). In the right hemisphere, other regions of increased perfusion were found in the right frontal medial region (Brodmann’s area 10 or 32), basal ganglia (putamen and globus pallidus), and inferior parietal lobule (Brodmann’s area 40). No significant decreases were found.
MR brain image shows areas of significantly increased perfusion. FLS = fasciculus longitudinalis superior; BA = Brodmann’s area.
Significant Areas of Increased Perfusion According to SPM Analysis
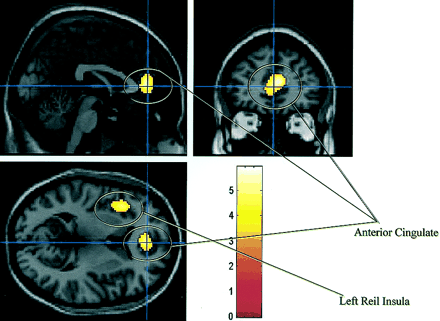
A simple regression analysis between the SPECT images for each subject (and condition) and the NPH scores showed a positive relationship in Brodmann’s area 32 (P corrected = 0.002; cluster = 412 voxels; coordinates = 6, 40, 12), corresponding to the anterior cingulate, and the frontal part of the left lobule of Reil insula (P corrected = 0.009; cluster = 289 voxels; coordinates = −37, 18, 3) (Fig. 3).
Image shows 2 areas most related to clinical improvement from regression analysis.
DISCUSSION
Using SPM analysis, this study showed significant areas of increased perfusion in specific brain regions of both frontal lobes and the right parietal lobe. This result was obtained with an image analysis method that does not require prior selection of regions of interest to characterize the postsurgical CBF changes. Despite the control for multiple comparisons, we found significant postsurgical changes in 6 brain regions located in the prefrontal, lenticular, and parietal areas. The finding of substantial frontal lobe involvement is consistent with previous studies that have also reported a predominant frontal CBF improvement after surgery (6,12,14). In fact, research from different approaches indicates that the frontal lobes are mainly involved in the pathophysiology of NPH. Neuropsychologic studies have found a pattern of cognitive deficits consistent with frontostriatal dysfunction (1,25–27). Neuroanatomic and neuroimaging studies also confirm and support this frontal lobe damage in patients with NPH. In a recent study by Klinge et al. (28) using quantitative H215O PET and SMP methodology, shunt responders showed significantly lower CBF values in the frontobasal cortex before surgery. Furthermore, in our study we found a right inferior parietal CBF improvement after surgery, corroborating the findings of Kimura et al. (14), who found CBF normalization in the white matter of the frontal and temporoparietooccipital lobes. Finally, we also found a significant relationship between SPECT images and the NPH scale in Brodmann’s area 32 and the frontal part of the left lobule of Reil insula. This suggests that these 2 areas are the cerebral regions that are most sensitive to the general clinical improvement in our patients with idiopathic NPH.
Predominant regions of increased perfusion in the left hemisphere were found in dorsolateral prefrontal areas and in the right hemisphere in the medial prefrontal region, frontal premotor area, basal ganglia, frontal white matter area, and inferior parietal lobe. Despite the bilateral involvement of the frontal lobes, the increased perfusion areas are not symmetric. Other studies have also found asymmetric CBF results in NPH patients. Mamo et al. (4) found an asymmetric preoperative reduction in 6 of 22 patients. In those subjects, they found a postsurgical higher increase on the side with the lower CBF. Meyer et al. (12) found decreased flow in the left frontal and left temporal white matter regions compared with the right. They suggested that the asymmetries might be related to the location of the shunt on the right-hand side. Two other CBF studies using an asymmetry index found heterogeneous flow patterns (10,11), and 2 other authors did not find any interhemispheric difference (15,29).
Divergences between previous CBF studies have been attributed to the difficulties in identifying suitable candidates with NPH and to the technical limitations of the methods used for measuring CBF (12,30). Another factor that could also explain the diversities between studies is the time interval between initial and follow-up SPECT evaluations. Motor and sphincter control recovery has been reported to be faster than cognitive improvement (31), which may occur as late as the second postoperative year (32,33). In our study, the 6-mo time interval might have detected the most significant cerebral changes, although we cannot rule out further recovery. Furthermore, NPH is a very heterogeneous condition. Waldemar et al. (10) suggested that the different abnormal CBF patterns found may reflect different stages of the disease or different underlying degenerative disorders. In a PET and histopathologic study, Tedeschi et al. (11) found heterogeneity in both the metabolic and histopathologic findings, also suggesting that NPH might be nonspecifically associated with different degenerative disorders.
Several hypotheses have been postulated to explain the decrease in CBF in NPH, which generally improves after surgery: microcirculation compression, CSF extravasation, and decreased metabolism (14). More recently, Bateman (34) has suggested that a reduction of craniospinal compliance is involved in the pathogenesis of NPH. According to this theory, a reduction of cortical vein compliance, associated with venous compression or structural venous changes, may increase resistance to blood flow at the superior sagittal sinus and cortical veins. After shunting, the reduction of cortical vein compliance can be reversed and the normal absorption pathway for CSF restored.
A limitation of our study is that no comparison with a healthy control population was performed at baseline. Therefore, no conclusions can be made regarding quantitative specific abnormalities in cerebral perfusion in NPH patients before surgery. Consequently, it is not possible to evaluate whether the increase in cerebral perfusion after surgery occurred in the regions that were previously affected. However, a visual semiquantitative inspection of the images of 20 patients that was performed in a SPECT study before and after surgery, as described in Díez-Castro et al. (35), revealed an overall reduction of brain uptake—mainly in frontal, parietal, and temporal lobes—with a significant improvement in postsurgical studies. After surgery, brain uptake improved in 80% of the patients.
CONCLUSION
The results obtained with the SPM method of image analysis confirm and expand the earlier CBF literature on NPH, with specific CBF regions located in frontal and parietal areas that improve after surgery in idiopathic NPH. Our results agree with previous studies with regard to the predominant involvement of frontal lobes in the pathophysiology of NPH. We provide new information suggesting the involvement of specific areas within the left prefrontal dorsolateral cortex, and within the right hemisphere in the medial frontal cortex, frontal premotor area, as well as in the basal ganglia, frontal white matter, and inferior parietal region. Further studies using this method on more patients with idiopathic as well as secondary hydrocephalus could shed further light on this still unknown and complex entity.
Acknowledgments
We thank Carles Falcon Falcon for his helpful comments and technical assistance. This article was partially supported by grants 99/0968 and 97/0923 from the Fondo de Investigación Sanitaria.
Footnotes
Received Apr. 4, 2003; revision accepted Aug. 14, 2003.
For correspondence or reprints contact: Carme Junqué, PhD, Institut d’Investigacions Biomèdiques August Pi-Sunyer, Casanova 143, 08036 Barcelona, Spain.
E-mail: cjunque{at}ub.edu