Abstract
Cardiac 123I-metaiodobenzylguanidine (123I-MIBG) uptake is reduced in chronic heart failure, and its reduction is reported to relate to the decrease in exercise capacity. Reduced 123I-MIBG uptake may predict an inadequately reduced adrenergic drive to the heart during cardiac sympathetic stimulation, including exercise. However, there is little information about the relationship between cardiac 123I-MIBG uptake at rest and norepinephrine (NE) release during exercise in relation to the exercise capacity in the failing heart. The aim of this study was to examine whether cardiac 123I-MIBG uptake at rest can predict cardiac sympathetic activity during exercise in patients with chronic heart failure. We determined how cardiac 123I-MIBG uptake at rest relates to NE overflow from the heart during symptom-limited graded exercise in such patients. Methods: Twelve patients (mean ± SD, 52 ± 12 y) with chronic stable heart failure performed symptom-limited graded exercise tests under catheterizations with a 4-min stage using a supine bicycle ergometer within 2 wk after 123I-MIBG imaging. NE concentrations in the arterial and coronary sinus blood (NEA and NECS, respectively) were measured at each exercise stage, and NE overflow was approximated by the difference between NECS and NEA (NECS−A). Results: The left ventricular ejection fraction at rest was 47% ± 16% and peak oxygen uptake was 17.7 ± 5.1 mL/kg/min. The heart-to-mediastinum uptake ratio of the delayed 123I-MIBG image (1.00∼1.72; mean ± SD, 1.30 ± 0.19) correlated with NECS−A at peak exercise (r = 0.80, P < 0.01) and peak heart rate (r = 0.73, P < 0.01) but not with peak oxygen uptake. Conclusion: Cardiac 123I-MIBG uptake of the delayed image can predict the degree of the increase in adrenergic drive to the heart during sympathetic stimuli induced by exercise in patients with chronic heart failure.
Noninvasive myocardial scintigraphy with 123I-metaiodobenzylguanidine (123I-MIBG), an analog of guanethidine that shares many neuronal transport and storage activities with norepinephrine (NE) (1–4), has been used to image efferent adrenergic terminals in the heart (5–8). Previous studies demonstrated a significant reduction of cardiac 123I-MIBG uptake in chronic heart failure (CHF), and the degree of reduction of 123I-MIBG uptake correlates with decreased exercise capacity (9–13) as well as resting left ventricular function (12,14) and prognosis (9–14).
If reduced 123I-MIBG uptake indicates a decrease in the total amount of functioning sympathetic nerve terminals in the failing heart, adrenergic drive to such failing heart may be inadequate, particularly during stimulation of cardiac sympathetic nerves. Exercise is the most powerful cardiac sympathetic stimulus, and increased adrenergic drive to the heart would play roles in cardiac responses during exercise and, subsequently, may do in exercise capacity. However, the relationship between 123I-MIBG uptake and effective cardiac sympathetic activity—that is, the amount of neurotransmitter NE derived from cardiac sympathetic nerves—has not been elucidated. Our hypothesis is that cardiac NE overflow during exercise is lower in CHF patients with reduced cardiac 123I-MIBG uptake. This hypothesis, if true, may shed new light on the mechanisms of reduced exercise tolerance in CHF with low cardiac 123I-MIBG uptake, and it may provide a basis for using cardiac 123I-MIBG uptake as a marker of cardiac sympathetic efferent function as well as that of reuptake function. Thus, we investigated the correlation of cardiac 123I-MIBG uptake at rest with NE overflow from the heart, which was approximated by the difference of NE concentration between coronary sinus and arterial blood (NECS−A) during symptom-limited graded exercise tests in patients with CHF.
MATERIALS AND METHODS
This clinical study was performed in accordance with the Declaration of Helsinki.
Subjects
Among consecutive patients who were admitted for a diagnosis and functional evaluation of heart failure, 12 patients without coronary artery disease and atrial fibrillation were enrolled in this study after diagnostic catheterization studies, including coronary angiography. The patient profiles are summarized in Table 1. The patients consisted of 9 men and 3 women, ranging in age from 27 to 70 y (mean ± SD, 52 ± 12 y). The criterion of CHF used in this study was the pathophysiologic state in which the heart cannot pump blood at a rate commensurate with the requirements of metabolizing tissues. According to the functional classification of the New York Heart Association, 2 patients were in class I, 8 in class II, and 2 in class III. Seven of the 12 patients had idiopathic dilated cardiomyopathy. Two patients had mild mitral stenosis and 1 of them had a percutaneous transluminal mitral commissurotomy 6 mo previously. Four of the 12 subjects (patients 1, 3, 9, and 11) had a left ventricular ejection fraction (LVEF) of >50%, indicating normal or mildly impaired systolic left ventricular function at rest. However, all of these 4 subjects had reduced peak oxygen consumption (Vo2) or cardiac index at peak exercise. Subjects 1 and 9 had diastolic dysfunction due to myocardial hypertrophy, 2 subjects (patients 3 and 11) had mitral stenosis, and 1 (patient 11) had a percutaneous transluminal mitral commissurotomy 6 mo previously. They had central hemodynamic abnormalities due to impaired cardiac pump function and had symptoms of heart failure for 3.0 ± 1.5 y before this study.
Profiles of Subjects
After entry into the study, within 2 wk, both 123I-MIBG scintigraphy and exercise tests with catheterization were performed on different days. Nine patients were receiving diuretics, 8 digitalis, and 10 an angiotensin-converting enzyme inhibitor on admission. Medication was withdrawn 24 h before the exercise test or 123I-MIBG scintigraphy. Informed consent was obtained from each subject after the nature and the purpose of the study was explained.
123I-MIBG Scintigraphy
Cardiac 123I-MIBG scintigraphy was performed in the morning after an overnight fast. Lugol solution (iodine, 40 mg/d) was administered orally from 3 d before to 3 d after the scintigraphic examination. After a 30-min resting period, 148 MBq 123I-MIBG (International CIS) was injected intravenously. At 30 min (early image) and 4 h (delayed image) after the injection, static acquisition was performed for 10 min in the anterior view of the chest, using a gamma camera (Toshiba 400T). An Elscint tomographic system with a general all-purpose collimator was used with 20% windows around the 159-keV photopeaks of 123I. Five-minute planar acquisition was performed in the anterior projection in a 256 × 256 matrix. For data processing, 123I-MIBG counts per minute were corrected for decay and were normalized to a 1-MBq dose. Whole left ventricular 123I-MIBG uptake was measured in a region of interest (ROI) drawn manually. Another manually drawn pixel ROI was placed over the middle mediastinum area. The heart-to-mediastinum (H/M) uptake ratio was then computed to quantify cardiac 123I-MIBG uptake. The washout rate was defined as the percentage change in the uptake within the ROI from the early to the delayed image.
Exercise Protocol
An 18-gauge polyethylene cannula was placed in the right brachial artery for monitoring the arterial pressure via a pressure transducer (Nihon Kohden AP-641G) and for taking blood samples. A 6 French Webster catheter was inserted about 10 cm into the coronary sinus through the right internal jugular vein. A 7.5 French balloon-tipped thermodilution catheter was inserted into the pulmonary artery for taking blood samples and measuring pressure and cardiac output. Continuous breath-by-breath respiratory gas analysis was performed during the test using a metabolic cart (model 3000; Minato Co.), and modified 12-lead electrocardiograms were also monitored continuously.
Blood sampling and hemodynamic measurements were performed twice with a 15-min interval before exercise and averaged for obtaining baseline resting values. Then, all patients performed a symptom-limited graded exercise with a 4-min stage using a supine bicycle ergometer. The workload was increased by 15–25 W according to individual exercise capacity. Cardiac output, aortic and pulmonary blood pressures, and heart rate were measured continuously, and blood gas was sampled between 3 and 4 min in each exercise stage. At the stage of the highest workload, blood samples were taken in the last minute after the patient’s perception of near-maximum.
Measurement of NE
Blood samples (10 mL) were withdrawn simultaneously into heparinized syringes from the brachial artery and coronary sinus and were transferred immediately into ice-cold tubes containing ethylenediaminetetraacetic acid. Samples were centrifuged at 3,000 rpm for 15 min at 4°C, and sera were separated and stored at −80°C. NE concentrations (pg/mL) in the arterial and coronary sinus blood (NEA and NECS, respectively) were determined by high-performance liquid chromatography (HPLC-725; TOSOH) using diphenylethylenediamine as a fluorogenic reagent (15). We obtained NECS−A, as the first-order approximation of cardiac NE overflow as described above.
Statistical Analysis
Data are expressed as mean ± SD. Intragroup comparisons were made with 2-tailed paired t tests. Associations between the plasma NE and 123I-MIBG H/M were examined by simple linear regression analysis. In addition, multiple stepwise regression analysis was performed to determine the interrelationship of the factors on cardiac 123I-MIBG uptake or hemodynamic variables. P < 0.05 was considered significant.
RESULTS
All subjects exercised up to their maximum work rate. Limiting symptoms were leg fatigue in 8 patients and shortness of breath in 4 patients. There were no complications during and after the exercise tests.
Resting Hemodynamics and Exercise Variables
As shown in Table 2, the LVEF by left ventriculography at diagnostic catheterization was 28%∼88% (mean, 47% ± 16%). The left ventricular end-diastolic volume index was 62∼243 mL/m2 (mean, 134 ± 61 mL/m2) and was inversely correlated with LVEF (r = −0.62, P < 0.05). Vo2 was 11.1∼30.9 mL/kg/min (mean, 17.7 ± 5.1 mL/kg/min), and the peak cardiac index was 4.4∼13.5 L/min/m2 (mean, 6.8 ± 2.6 L/min/m2). There was a significant positive linear correlation between the peak Vo2 and the peak cardiac index (r = 0.74, P < 0.01), whereas LVEF at rest did not correlate with either peak Vo2 or the peak cardiac index. Blood lactate and plasma NE of arterial blood were 0.8 ± 0.2 mmol/L and 88 ± 54 pg/mL at the baseline resting state and 4.4 ± 1.8 mmol/L and 609 ± 197 pg/mL at the peak exercise stage (P < 0.01 vs. baseline, each), respectively.
Indices of Cardiac 123I-MIBG Uptake and Left Ventricular Function at Rest and NE and Exercise Variables at Peak Exercise
Relationships Between NE Overflow and 123I-MIBG Uptake
A representative case with high NE overflow and only mildly reduced cardiac 123I-MIBG uptake (subject 1) is shown in Figure 1. NE concentrations in coronary sinus venous blood increased more than those in arterial blood with respect to Vo2 (Figs. 1A and 1B). During exercise, NECS−A increased linearly with respect to NEA in this case (Fig. 1C). Figures 1D and 1E show 123I-MIBG images in this case, demonstrating only mildly reduced cardiac 123I-MIBG uptake. The H/M ratios at the early and delayed images were 1.76 and 1.72, respectively. In contrast, a case with low NE overflow and low cardiac 123I-MIBG uptake (subject 4) is shown in Figure 2. NECS increased less than NEA with respect to Vo2 in this case (Figs. 2A and 2B). During exercise, NECS−A decreased linearly with respect to NEA (Fig. 2C). Cardiac 123I-MIBG activity was barely observed in this case (Figs. 2D and 2E), and the H/M ratios at the early and delayed images were 1.09 and 1.09, respectively.
(A) Changes in NE concentrations at coronary sinus (CS) and brachial artery (artery) with respect to Vo2 during graded exercise in representative case with positive NE overflow. (B) Changes of difference in NE concentration between coronary sinus and arterial blood (NECS−A) with respect to Vo2. (C) Changes in NECS−A with respect to arterial NE concentration (NEA). (D and E) Initial (D) and delayed (E) 123I-MIBG planar images in anterior view. Cardiac 123I-MIBG activity was reduced only mildly. H/M = 123I-MIBG uptake ratio.
(A) Changes in NE concentrations at coronary sinus (CS) and brachial artery (artery) with respect to Vo2 during graded exercise in representative case with negative NE overflow. (B) Changes of NECS−A with respect to Vo2. (C) Changes in NECS−A with respect to arterial NE concentration (NEA). (D and E) Initial (D) and delayed (E) 123I-MIBG planar images in anterior view. Cardiac 123I-MIBG activity was barely recognized. H/M = 123I-MIBG uptake ratio.
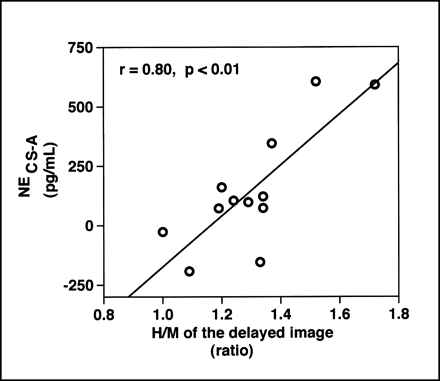
The absolute value of the correlation coefficient between NECS−A and NEA was 0.61∼0.99 in all cases (0.84 ± 0.13, P < 0.05 in all cases in Table 2). Although the relationship between NECS−A and NEA was linear in the range from the resting state to the peak exercise stage in each subject, among the 12 subjects NECS−A did not correlate with NEA, both at the baseline resting state and at peak exercise, but it did correlate with NECS, both at rest (r = 0.81, P < 0.01) and at peak exercise (r = 0.84, P < 0.01) by simple linear regression analysis. The H/M of the 123I-MIBG delayed image did not correlate with NEA, both at the baseline resting state and at peak exercise, but it did correlate with NECS−A, both at rest (r = 0.62, P < 0.05) and at peak exercise (r = 0.80, P < 0.01; Fig. 3).
Scatter diagram illustrating relationship between H/M of delayed image and NECS−A at peak exercise. There was positive linear relationship between them (r = 0.80, P < 0.01).
By multiple stepwise regression analysis, there was a positive correlation of the NECS−A and H/M of the 123I-MIBG delayed image and the cardiac index at peak exercise (H/M of delayed image = 1.03 + 0.001 × [NECS−A at peak exercise] + 0.03 × [cardiac index at peak exercise]; P = 0.0003).
Relationships Between 123I-MIBG Imaging or NE Concentration and Hemodynamic Variables at Resting State and Peak Exercise
By simple linear regression analysis, there were no significant relationships between the indices of 123I-MIBG imaging—that is, the H/M of early or delayed images, or the washout rate, and any exercise variables such as Vo2, cardiac index, and heart rate at peak exercise, except that the H/M of the delayed image correlated with the peak heart rate (r = 0.73, P < 0.01) and NECS−A (r = 0.63, P < 0.05) and NECS at peak exercise also correlated with he peak heart rate (r = 0.63, P < 0.05). However, none of them correlated with other variables at rest and peak exercise. Indices of 123I-MIBG imaging and NECS−A at the baseline resting state did not correlate with the LVEF, left ventricular end-diastolic volume index, cardiac index, or other resting hemodynamic variables. By multiple stepwise regression analysis, the cardiac index at peak exercise was positively correlated with Vo2 at peak exercise in association with negatively correlated NEA at the resting state (cardiac index at peak exercise = 0.92 + 0.47 × [Vo2 at peak exercise] − 0.02 × [NEA at rest]; P = 0.0007).
DISCUSSION
Cardiac 123I-MIBG uptake of the delayed image correlated with NE overflow and peak heart rate during symptom-limited graded exercise tests, although it did not correlate with peak Vo2. This suggests that adrenergic drive—that is, exposure of the heart to NE, during cardiac sympathetic stimulation induced by exercise—is reduced in patients with decreased cardiac 123I-MIBG uptake at rest, and vice versa.
NE is released from sympathetic nerve terminals into the neural cleft with impulse traffic in efferent postganglionic sympathetic nerves, and then a large fraction of the released NE is actively taken up and stored in sympathetic nerve terminals, and 123I-MIBG uses the same uptake system and storage sites as NE (1–4). In animal experiments, the H/M of the delayed 123I-MIBG image was reduced in pacing-induced heart failure and it was correlated with global and regional NE content of the left ventricle (8). Moreover, that study suggested that 123I-MIBG uptake estimates the stored content of NE available for release in the sympathetic nerve terminals and, accordingly, the density of functioning sympathetic neurons. Our results broaden this concept and show that the H/M of the delayed 123I-MIBG image could also indicate the NE reserve for release by cardiac sympathetic nerves when stimulated by exercise in CHF. In this study, the NECS−A correlated with the H/M of the delayed image but not with the H/M of the early image and the washout rate. It is known that the nonneuronal uptake of 123I-MIBG is significant in the early phase after injection and is decreased 3–5 h later (7,16). For this reason, the delayed 123I-MIBG uptake has been considered an index of the degree of neuronal accumulation of the tracer (17). Previous studies showed that the H/M of the 123I-MIBG delayed image is a more useful index of the severity and prognosis of CHF than the H/M of the early image or the washout rate (9–14).
It is known that NECS−A increases in parallel with exercise intensity in healthy subjects (18,19), but NECS−A during exercise has not been examined in patients with CHF. NECS−A would reflect the total amount of NE overflow from sympathetic synaptic neural clefts. NE that overflows into the circulation is the fraction of NE released from the nerve terminals that has escaped neuronal and extraneuronal uptake. Therefore, NECS−A would be dependent on the functioning sympathetic neurodensity in the heart and on the release and reuptake of NE. In addition, it would be dependent on coronary blood flow, although the rate of increase in coronary blood flow during exercise has been reported to be similar in patients with CHF and in healthy subjects (18). The failing myocardium is characterized by many alterations of adrenergic nerves, such as partial sympathetic denervation and decreased NE content (8), increased NE release due to a persistently activated sympathetic center (20,21), enhancement by neurohormonal factors such as tissue angiotensin II (22), and depressed activity of the sympathetic neuronal NE uptake system(s) (23). Each factor, in association with other factors, may have had a role in the decreases in NE overflow and 123I-MIBG uptake, although their relative contributions to them was not determined in this study.
A recent report showed that reinnervated cardiac sympathetic nerve density after heart transplantation, identified by the activity of the catecholamine analog 11C-hydroxyephedrine, correlated with chronotropic and left ventricular contractile responses to exercise and with peak oxygen uptake (24). This suggested a significant role for cardiac sympathetic nerves in cardiac responses during exercise and, subsequently, exercise capacity. However, effector cardiomyocytes may be intact in the transplanted heart. Therefore, the relationship between adrenergic drive and cardiac inotropic and chronotropic responses would be very different from that in CHF, in which cardiomyocytes are injured and β-receptors are desensitized (25). In addition, dysfunction of peripheral vascular beds, rather than cardiac dysfunction, is considered to be a main determinant of exercise capacity in CHF (26,27). Therefore, it would not necessarily be expected that the amount of cardiac adrenergic drive is correlated with exercise capacity in CHF. In this study, the adrenergic drive to the heart expected from NECS at peak exercise, and indirectly estimated by the H/M of the delayed 123I-MIBG image, was significantly correlated with peak heart rate. Reduced chronotropic reserve in CHF has been considered to be due mainly to β-adrenergic desensitization of the sinus node rather than to altered NE kinetics (25). However, in the patients with CHF assessed in this study, NE concentration in the sinus node seemed to play a role.
There are some limitations to this study. First, NECS−A examined in this study is a first-order approximation of the whole cardiac sympathetic activity and does not mean the net amount of NE overflow from the heart. The method using infusion of 3H-NE is generally accepted as a standard for determining the rates of NE spillover from individual organs and its clearance (19,21,22). However, this method requires steady-state exercise, and in this study it was not possible to assess the full-range of responses up to the maximal exercise intensity. Second, this was a study of a small population of patients with different underlying diseases except for ischemic heart disease, and healthy subjects were not enrolled because of the invasiveness of the hemodynamic study and exercise test. Nevertheless, it may be important from a clinical aspect that the correlation between the cardiac NE overflow during exercise and the cardiac 123I-MIBG uptake on delayed image was observed consistently despite the inhomogeneity of our study population. Third, the exact mechanism of reduced NE overflow during exercise in the heart with reduced 123I-MIBG uptake needs to be determined to see whether reduced NE overflow during exercise may mean impairment of cardiac sympathetic efferent function in addition to impairment of uptake-1. Considering these limitations, to enhance the overall significance of this study, further studies will be required on the relation of cardiac 123I-MIBG uptake, the exact mechanisms of cardiac sympathetic dysfunction, and their clinical overall relevance.
CONCLUSION
Cardiac 123I-MIBG uptake of the delayed image in the resting state could predict the degree of substantial adrenergic drive during cardiac sympathetic stimulation by exercise in patients with CHF. Therefore, 123I-MIBG imaging can provide important information about NE in adrenergic drive—that is, exposure of the heart to NE when cardiac sympathetic nerves are stimulated by exercise.
Footnotes
Received Jan. 28, 2003; revision accepted May 20, 2003.
For correspondence or reprints contact: Yukio Maruyama, MD, First Department of Internal Medicine, Fukushima Medical University, Hikarigaoka 1, Fukushima, 960-1295, Japan.
E-mail: maruyama{at}fmu.ac.jp