Abstract
Left ventricular (LV) systolic function in hypertrophic cardiomyopathy (HCM) is usually normal. Late in the disease, however, LV systolic dysfunction and dilatation are recognized. Although abnormalities in cardiac sympathetic nerve activity in patients with HCM have been demonstrated using 123I-metaiodobenzylguanidine (MIBG) myocardial scintigraphy, the changes of cardiac sympathetic nerve activity throughout the clinical course from typical to end-stage HCM are unclear. The objective of this study was to evaluate the relationship between abnormalities on 123I-MIBG myocardial scintigraphy and pathophysiologic changes in patients with HCM. Methods: We performed 123I-MIBG scintigraphy on 46 patients with HCM and 18 age-matched control subjects. The patients were categorized into 3 groups: 28 patients with normal LV systolic function (group A), 9 patients with LV systolic dysfunction (group B), and 9 patients with LV systolic dysfunction and dilatation (group C). With planar 123I-MIBG imaging, the heart-to-mediastinum ratio for early and delayed acquisitions and the washout rate were calculated. With SPECT, polar maps of the LV myocardium were divided into 20 segments. The regional uptake and washout rate were calculated from semiquantitative 20-segment bull’s-eye analysis. Results: The early uptake was significantly lower in group C than in the control group (P < 0.01). The washout rate was progressively higher in group A, group B, and group C (P < 0.01). Reduced regional early uptake was found in 2.9 ± 3.4 (group A), 4.1 ± 4.7 (group B), and 7.4 ± 4.3 (group C) segments, respectively. In group C, regional early uptake was significantly reduced, predominantly in the interventricular septal wall, and regional washout rate was increased in the apex and lateral wall. Conclusion: These results suggest that cardiac sympathetic nerve abnormalities in patients with HCM may advance with development of LV systolic dysfunction and dilatation and that 123I-MIBG myocardial scintigraphy may be a useful tool for the evaluation of pathophysiologic changes in HCM.
- hypertrophic cardiomyopathy
- 123I-metaiodobenzylguanidine
- sympathetic nerve activity
- pathophysiologic changes
Hypertrophic cardiomyopathy (HCM) is a disease characterized by disproportionate left ventricular (LV) hypertrophy and LV diastolic dysfunction. LV systolic function in HCM is usually normal or supernormal (1). The clinical course and prognosis in patients with HCM are characterized by sudden death associated with life-threatening arrhythmias, by LV diastolic dysfunction, and by stroke associated with atrial fibrillation (2). Late in the disease, however, impaired LV systolic function and LV dilatation are recognized in some patients with HCM (2,3). Consequently, these patients whose disease has evolved into the end stage are at high risk of progressive congestive heart failure (2,3). Nevertheless, the characteristics of end-stage HCM have been studied only rarely.
Cardiac nerve activity plays an important role in the modifications of cardiac function that contribute to such disease processes as heart failure (4). Recently, 123I-metaiodobenzylguanidine (MIBG), an analog of norepinephrine, has been used to evaluate cardiac sympathetic nerve activity in various kinds of pathologic conditions (5–8). HCM is reported to involve dysfunctional sympathetic innervation (9,10). However, the changes in cardiac sympathetic nerve activity throughout the clinical course from typical to end-stage HCM are unclear. The present study was performed to evaluate the relationship between abnormalities on 123I-MIBG myocardial scintigraphy and pathophysiologic changes in patients with HCM so that the clinical course of and mechanisms leading to LV systolic dysfunction and dilatation can be further understood.
MATERIALS AND METHODS
Patients with HCM
Before the study, informed consent was obtained from all subjects. The study included 46 patients with HCM (30 male and 16 female; mean age, 52.7 ± 13.1 y). The diagnosis of HCM was based on the typical clinical findings of unexplained cardiac hypertrophy and a maximum LV wall thickness (WT) ≥ 13 mm at some time during the clinical course (11). LV systolic dysfunction was defined as LV fractional shortening (FS) < 25%, and LV dilatation was defined as LV end-diastolic dimension (EDD) ≥ 55 mm on echocardiography. At the time of the 123I-MIBG scintigraphic study, the patients with HCM were categorized into 3 groups: group A, with normal LV systolic function (n = 28; WT ≥ 13 mm, FS ≥ 25%, and EDD < 55 mm); group B, with LV systolic dysfunction (n = 9; WT ≥ 13 mm, FS < 25%, and EDD < 55 mm); and group C, with LV systolic dysfunction and dilatation (n = 9; WT ≥ 13 mm, FS < 25%, and EDD ≥ 55 mm). Although the WT in 1 patient in group B and 3 patients in group C was <13 mm, they were also enrolled in the study because they had a family history of HCM and the same gene mutation as the proband with HCM. Patients with myocardial infarction, valvular heart disease, hypertension, or diabetes mellitus were excluded from the study.
Control Subjects
As a control group, 18 subjects with no evidence of heart disease possibly affecting sympathetic nervous activity were investigated. There were 7 men and 11 women ranging in age from 27 to 74 y (mean age, 52.9 ± 15.4 y).
Evaluation of Cardiac Sympathetic Nerve Activity
No subjects were taking reserpine or tricyclic antidepressants at the time of the study. The method of 123I-MIBG scintigraphy has been published previously (12, 13). In brief, each subject was administered an intravenous injection of 120–140 MBq of commercially available 123I-MIBG between 9:00 and 10:00 am. A 5-min static acquisition was made in the anterior view at 20 min (early) and 3 h (delayed) after the injection of 123I-MIBG. Projection images were acquired after each static acquisition with a 3-head scintillation camera (model 9300A; Toshiba) equipped with parallel-hole, high-resolution collimators. The energy discrimination was centered on 159 keV with a 20% window. Sixty projection images were obtained in a 64 × 64 matrix over a 360° arc with 30-s acquisitions for each view. Tomographic images were reconstructed using a Butterworth filter with a cutoff frequency of 0.47 cycle/cm and an order of 8.
Three nuclear medicine experts who were unaware of other clinical findings analyzed the data by consensus. On the anterior planar 123I-MIBG images, the rectangular region of interest (ROI) was manually drawn over the whole heart, including LV cavity, on the upper mediastinum. The heart-to-mediastinum ratios for the early and delayed images were calculated to quantify cardiac 123I-MIBG uptake as a fraction of the mean count per pixel in the heart ROI divided by that in the upper mediastinum ROI. The washout rate was calculated as follows: washout rate = (Ec–Dc) × 100/Ec, where Ec is early cardiac count density and Dc is decay-corrected delayed cardiac count density.
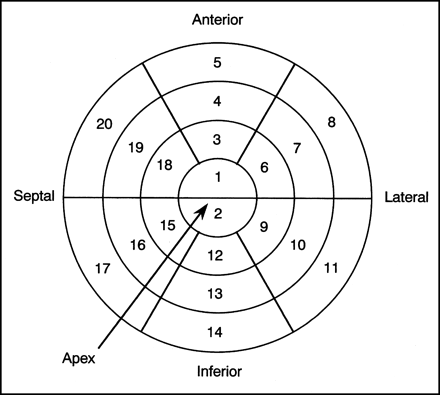
We evaluated nongated 123I-MIBG images using Quantitative Gated SPECT software (version 2; Cedars-Sinai Medical Center) (14). The regional uptake and washout rate were calculated using semiquantitative 20-segment bull’s-eye analysis, which has been previously described (12,13). In brief, polar maps of all subjects were divided into 20 segments as shown in Figure 1. The rectangular ROI was placed on each of the 20 segments. The percentage uptake in the early image and the washout rate in each segment were obtained. An abnormal reduction in regional 123I-MIBG uptake was defined as uptake that was 2 SDs less than the mean value of the same segment in the control group.
Polar map of LV myocardium was divided into 20 segments as shown, and uptake and washout rate in each section were determined.
Echocardiography
All patients underwent echocardiography by experienced operators within 1 wk of the 123I-MIBG myocardial scintigraphy. Measurements of LV dimensions, the thickness of the interventricular septum, and the thickness of the LV posterior wall were taken at the level of the tips of the mitral valve leaflets in the parasternal long-axis view. The FS was calculated as the difference in end-diastolic and end-systolic dimensions divided by the EDD.
Statistical Analysis
Continuous data are presented as mean ± SD, and categoric data are presented as relative frequency (percentage). For semiquantitative analysis of 123I-MIBG scans, each of the 20 segments of the polar map was compared with the corresponding segment in the control group. Paired mean values were compared using 1-way ANOVA followed by the Scheffé multiple-comparisons test. Categoric data were compared using the χ2 test. P < 0.05 was considered statistically significant.
RESULTS
Baseline Characteristics of Study Patients
The baseline clinical characteristics of the patients are summarized in Table 1. No significant differences in sex or age existed among the 3 groups. New York Heart Association functional class was significantly and progressively higher in group A, group B, and group C. Echocardiography showed that interventricular septal thickness and maximum LV WT were significantly thinner, LV EDD and LV end-systolic dimensions were significantly larger, and LV FS was significantly lower in group C than in either group A or group B. The other echocardiographic parameters were comparable across groups.
Baseline Characteristics of Patients with HCM
123I-MIBG Scintigraphic Data
123I-MIBG scintigraphic data are summarized in Table 2. Regarding global 123I-MIBG parameters, the early and delayed uptake ratios for the heart and mediastinum were significantly lower in group C than in the control group. Washout rate was significantly and progressively higher in the control group, group A, group B, and group C, as shown in Figure 2.
Early uptake ratio and washout rate of 123I-MIBG in control group and in groups of patients with HCM.
123I-MIBG Scintigraphic Data
Regarding regional 123I-MIBG parameters, the number of segments with reduced early uptake was significantly greater in group C than in group A (P < 0.01). The number of segments with an increased washout rate tended to increase progressively in group A, group B, and group C, but the increases were not statistically significant.
Compared with the corresponding segments in the control group, no segments in group A showed significantly reduced regional early uptake, and only a few segments attained significance in group B. In contrast, regional early uptake was significantly reduced in the interventricular septal wall, predominantly in group C (Fig. 3). In addition, the regional washout rate in group C was significantly increased in the apex and lateral wall, whereas in group A this factor was not significantly increased in any of the segments (Fig. 4).
Polar maps depicting differences in relative 123I-MIBG early uptake between groups of patients with HCM. Ant = anterior wall; Lat = lateral wall; Post = posterior wall; Sept = interventricular septum.
Polar maps depicting differences in relative 123I-MIBG washout rate between groups of patients with HCM. Ant = anterior wall; Lat = lateral wall; Post = posterior wall; Sept = interventricular septum.
DISCUSSION
HCM is a primary myocardial disease with a wide clinical spectrum and marked variability in pathophysiology. Although recent studies provide evidence of abnormal cardiac autonomic innervation in patients with HCM (6,13–16), we are aware of no published reports that describe the changes of cardiac sympathetic nerve activity—in particular, regions of damage—throughout the clinical course from typical to end-stage HCM. We reported previously that the heterogeneity of cardiac sympathetic nerve activity correlated with cardiac function in patients with HCM (14). In the current study, we evaluated in more detail the relationship between abnormalities on 123I-MIBG myocardial scintigraphy and the pathophysiologic changes in patients with HCM. The major findings of the study were, first, that cardiac sympathetic denervation measured by the early uptake of 123I-MIBG is vague or nonexistent in HCM patients with normal LV systolic function but eventually occurred in the end stage characterized by LV systolic dysfunction and dilatation; second, that cardiac sympathetic nerve hyperactivity measured by washout rate had already occurred in the stage of normal LV systolic function in HCM and increased with the progression to the end stage; and third, that especially in end-stage HCM, regional sympathetic denervation was shown predominantly in the interventricular septal wall, as well as in the apex and the anterior and posterior walls. In contrast, regional sympathetic nerve hyperactivity was recognized in the apex and apical lateral wall.
These findings might be related to histopathologic changes in HCM. HCM is a disease caused by a mutation in the genes encoding sarcomere proteins such as β-myosin heavy chain, cardiac troponin T, and cardiac troponin I (17–19). Mutated proteins and the myocyte disarray characterizing HCM may decrease the function of the myocardial contractile unit. In response to this dysfunction, sympathetic nerve activity may be increased and thus may lead to myocyte hypertrophy resulting in normal systolic function of the global left ventricle. Considering these mechanisms, in the patients of group A, with normal LV systolic function, early uptake of 123I-MIBG might not differ from the values in control subjects, and the washout rate of 123I-MIBG might be increased in comparison with control values.
In contrast to the histopathologic changes in HCM, development of LV systolic dysfunction during the transition from typical HCM with wall hypertrophy to end-stage HCM is related to thinning of the interventricular septal wall (20). Histopathologic examinations revealed extensive myocardial fibrosis in the thin septal wall of the end-stage HCM heart (21). These findings led us to hypothesize that development of LV systolic dysfunction caused by myocyte death and increased fibrosis in the septal wall leads to an increase in sympathetic nerve activity resulting in a further increase in washout of 123I-MIBG. When these histopathologic changes progress, few myocytes and extensive fibrosis exist in the septal wall, and LV systolic dysfunction and dilatation develop. Consequently, early uptake of 123I-MIBG is decreased in the septal wall. In response to decreased cardiac function, sympathetic nerve activity in the apex and lateral wall, where myocytes are viable, increases, thus preserving LV function. Our hypothesis may account for the indications that early uptake of 123I-MIBG was decreased in the septal wall and that the washout rate of 123I-MIBG was increased in the apex and lateral wall in group C, with LV systolic dysfunction and dilatation.
However, we acknowledge that the small sample size limits our study. We recognize that to include a larger number of patients in future studies would be optimal. However, the current data are important in determining the possible role cardiac sympathetic nerve activity plays in evaluating pathophysiologic changes in HCM.
In this study, we investigated HCM by using regional parameters in addition to global parameters for a more accurate comparison. There were a few discrepancies between the results of global and regional parameters such that no segment of increased washout rate was found in group A. One possible reason for this finding may be heterogeneous sympathetic nerve activity based on heterogeneous wall hypertrophy in HCM. Because global parameters have limitations due to lung overlap, evaluation of regional washout rate might provide more accurate information than evaluation of global washout rate. On the other hand, regional early uptake is relative to percentage uptake. If there are regions of increased uptake, one may erroneously conclude that decreased uptake is present in a region that actually has normal uptake. If new technology for the assessment of absolute regional uptake is developed, more accurate information will be available.
Several metabolic nuclear imaging studies have demonstrated evidence of myocardial ischemia in patients with HCM (22–24). In addition, increased WT in intramyocardial coronary arteries is found in the interventricular septum in patients with end-stage HCM (21,25). Because cardiac sympathetic nerves are more sensitive to ischemia than are myocytes (26,27), myocardial ischemia may also play an important role in the changes in regional cardiac sympathetic nerve activity in HCM. However, the methods of the present study could not discriminate between the influence of ischemia and the influence of pathologic changes on cardiac sympathetic nerve function in HCM. For that purpose, other assessments, such as the combination of 123I-MIBG and sestamibi myocardial scintigraphies, may be useful.
HCM is the most common cause of cardiac-related sudden death in people younger than 30 y (28). However, a recent report on natural history indicates that progression of HCM to LV dilatation and dysfunction occurs in 10%–15% of patients (2,3,29). In addition, Seiler et al. found that patients with LV dilatation have a worse prognosis than those without, particularly regarding quality of life (30). We expect that our results will improve understanding of the process to end-stage HCM and be useful in the management of patients with HCM.
CONCLUSION
The results from our study suggest that abnormalities of cardiac sympathetic nerve activity in patients with HCM may advance with the development of LV systolic dysfunction and dilatation and that 123I-MIBG myocardial scintigraphy may be a useful tool for evaluating pathophysiologic changes in HCM.
Acknowledgments
We are indebted to Tomohito Mabuchi, Tetsuo Konno, and Tomoya Kaneda for technical support and analyses.
Footnotes
Received Mar. 10, 2003; revision accepted Jun. 14, 2003.
For correspondence or reprints contact: Hidenobu Terai, MD, Molecular Genetics of Cardiovascular Disorders, Division of Cardiovascular Medicine, Graduate School of Medical Science, Kanazawa University, Takara-machi 13-1, Kanazawa 920-8640, Japan.
E-mail: yonken1{at}med.kanazawa-u.ac.jp