Abstract
Most first relapses in patients with melanoma occur in regional lymph node basins. Such lesions are frequently diagnosed clinically during the first 2 y of follow-up. In the last few years, our group has been studying the usefulness of 99mTc-methoxyisobutylisonitrile (MIBI) scintigraphy in the evaluation of recurrent melanoma lesions. The aim of the present study was to prospectively evaluate the clinical value of 99mTc-MIBI scintigraphy in the diagnosis of subclinical nodal metastases. Methods: We included 66 patients within 3 mo of melanoma diagnosis, with Breslow thickness > 1.0 mm, all treated with wide local excision of the primary lesion. When 99mTc-MIBI scanning was performed, 49 of them did not have evidence of nodal disease, and 17 had clinically questionable regional lymph node lesions. Planar images of lymph node regions were acquired 10 min after injection, using a dose of 740–1,110 MBq and a large-field-of-view gamma camera equipped with a low-energy high-resolution collimator. Scan findings were confirmed by pathology or by clinical follow-up (median, 35 mo). Results: Thirty of 33 patients with regional lymph node metastases received a correct diagnosis, 14 with palpable lesions and 16 with nonpalpable lesions. In 3 cases that were initially 99mTc-MIBI negative, nodal metastases were found during follow-up. The following diagnostic values were calculated: sensitivity, 0.91 (95% confidence interval [CI], 0.75–0.98); specificity, 0.85 (95% CI, 0.67–0.94); likelihood ratio of a positive test, 6.0 (95% CI, 2.7–13.5); and likelihood ratio of a negative test, 0.11 (95% CI, 0.036–0.32). Conclusion: 99mTc-MIBI scanning may have a secondary role in the staging of regional lymph nodes in patients with clinically localized melanoma who are not good candidates for sentinel node biopsy.
Early diagnosis of regional lymph node metastases is a key step in the management of patients with melanoma. This information is relevant for the identification of patients who are candidates for adjuvant therapy with interferon α-2b and helps determine whether early therapeutic lymph node dissection is indicated (1).
Several standard imaging techniques, including ultrasound, MRI, CT, and conventional radiographic examination, rely on enlargement of lymph nodes, but enlarged nodes are not necessarily malignant, and normally sized nodes may contain malignant cells (2,3). Radiocolloid lymphoscintigraphy combined with intraoperative probe detection of the sentinel node has become widely accepted for staging regional lymph nodes and is the standard of care in many major melanoma centers (4,5). Positive findings on sentinel node biopsy have been found to carry greater prognostic significance than does Breslow thickness. However, some authors have argued that unless the use of sentinel lymph node biopsy can be shown to improve overall survival, it should be performed only in the context of a clinical trial (6).
More recent interest has been directed toward “metabolic imaging” by 18F-FDG PET before lymph node metastases become clinically evident (3,7,8). However, other nuclear medicine techniques such as 67Ga scintigraphy or immunoscintigraphy applying monoclonal antibodies directed against melanoma-associated antigens have been used without offering any significant advantage in diagnostic sensitivity (9,10).
In the last few years, our team has been studying the clinical value of 99mTc-methoxyisobutylisonitrile (MIBI) scintigraphy for the evaluation of metastatic melanoma lesions. The technique proved to have the potential of detecting subclinical recurrent disease, including lymph node metastases (11,12). In this study, we undertook a prospective investigation of patients with a recently diagnosed melanoma to assess the clinical value of 99mTc-MIBI scanning for the detection of regional lymph node metastases.
MATERIALS AND METHODS
Patients
The study was organized as a prospective nonrandomized trial, with 66 patients being consecutively enrolled from December 1997 to April 2001 according to a protocol approved by the local ethics committee.
After giving informed consent, 36 women and 30 men who had a histologically proven cutaneous melanoma and were within 3 mo of the initial diagnosis were entered into the study. Study entry occurred either before (n = 46) or after (n = 20) wide local excision of the primary lesion. The patients had to be at least 18 y old. Their median age was 54 y (range, 21–88 y). Breslow thickness had to be greater than 1.0 mm, and the clinical stage had to be IB–II (n = 49) or III (n = 17) according to the American Joint Committee on Cancer (AJCC). In this last subgroup, we included only patients with clinically questionable regional lymphadenopathies. Exclusion criteria were as follows: previous sentinel or complete lymphadenectomy, clinical or radiologic evidence of distant metastases, infection or inflammation in the regional node basin, prior fine-needle aspiration or excisional biopsy of regional lymph nodes, lack of histopathologic report of primary lesion, pregnancy or breast-feeding, and history of prior malignancy.
At entry, the histologic findings of the primary tumors were noted (e.g., location of the melanoma, thickness according to the method of Breslow, and histologic tumor type) (Table 1). The lymph node basins of all patients were clinically examined by an experienced clinician initially and at 4- to 6-mo intervals during follow-up until regional lymph node relapse was confirmed or the study closed (October 20, 2001). Median follow-up was 35 mo, with a range of 6–46 mo. All patients were treated with wide local excision of the primary lesion. Excisional lymph node biopsies were performed after the finding of a palpable lymphadenopathy on clinical examination at initial staging or during follow-up. Furthermore, therapeutic lymphadenectomy was indicated in those patients with histologically confirmed lymph node metastases. Patients with a diagnosis of nodal metastases had evidence of disease in 1 lymph node basin.
Clinical Stage and Characteristics of Primary Tumors in 66 Melanoma Patients
99mTc-MIBI Scintigraphy
Imaging started 10 min after an intravenous injection of 740–1,110 MBq of 99mTc-MIBI. The radiopharmaceutical was injected into an antecubital vein or the dorsal pedis vein, depending on the topography of the primary lesion, to avoid nonspecific accidental tracer accumulation in regional lymph nodes because of extravasation at the injection site. The equipment consisted of a large-field-of-view rectangular gamma camera (Sophy DSX; Sopha Medical) fitted with a low-energy, high-resolution collimator. Planar images of regional lymph nodes were acquired on a 256 × 256 matrix, using a 10% window centered on an energy peak of 140 keV. We used an isotime acquisition of 10 min for each view. The following regions were scanned depending on the primary-lesion location: head and neck (anterior and lateral views with extended neck), axillary region (anterior thoracic view with arms raised behind the head), and inguinal region (anterior pelvic view with previous voiding). Additionally, a whole-body acquisition with a scan speed of 16 cm/min was performed immediately afterward.
The 99mTc-MIBI scans were read by 3 experienced nuclear physicians knowing only the location of the melanoma, who reached a final consensus on the interpretation. A lesion was defined as a focus of increased 99mTc-MIBI uptake, compared with the intensity of surrounding activity, in a lymph node basin. Imaging was performed at a mean of 5 d (range, 1–7 d) after the initial clinical examination of lymph nodes.
Data Analysis
A focus of abnormal 99mTc-MIBI uptake in regional lymph nodes was defined as a metastasis and thus as true-positive if confirmed by histology. 99mTc-MIBI findings were true-negative if they were normal and the regional lymph nodes were histologically benign or the clinical course was benign, with no evidence of regional lymph node metastases. 99mTc-MIBI findings were false-negative if they were normal but regional lymph node histology revealed metastasis from melanoma. 99mTc-MIBI findings were false-positive if they showed a focus of abnormality but lymph node histology was benign or there were no clinical signs of regional lymph node metastases at follow-up.
Data were collected on case report forms, entered into a database, and converted to appropriate software datasets for analysis. Agreement between 99mTc-MIBI scintigraphy and histopathology or clinical course was assessed on a 2 × 2 table, which was the gold standard. Sensitivity and specificity and their corresponding 95% confidence intervals were calculated with InStat (version 3.01; GraphPad Software, Inc.) for Windows 95 (Microsoft). This software was also used for performing the Fisher exact test to compare 99mTc-MIBI results in different subsamples. Group differences were considered significant at P < 0.05.
The probability of regional lymph node metastases given the result of the 99mTc-MIBI scan was calculated using Arcus Quickstat (version 1.2; Longman Software Publishing) for Windows 95 as follow: LRpos = sensitivity/1 –specificity, where LRpos is the likelihood ratio of a positive test, and LRneg = 1 –sensitivity/specificity, where LRneg is the likelihood ratio of a negative test. The corresponding 95% confidence intervals were also estimated.
RESULTS
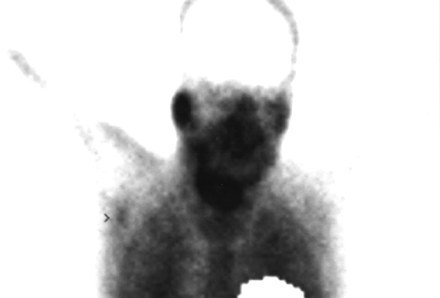
In 35 patients, a total of 35 regional lymph node sites of abnormal 99mTc-MIBI accumulation were identified, 30 of which proved to be melanoma metastases confirmed by cytology or histology. Five abnormal foci were shown to be false-positive. Four of these were in male patients with abnormal tracer uptake in the groin region who remained free of disease for 35–45 mo. The remaining patient had palpable axillary lymph nodes that were further classified as inflammatory lesions by pathology (Fig. 1).
Anterior thoracic view of patient with T2b melanoma of trunk shows focal 99mTc-MIBI uptake in left axilla (arrow), corresponding to palpable lymphadenopathy that proved to be inflammatory by histology.
The technique detected 30 of 33 (91%) confirmed regional lymph node metastases (Figs. 2 and 3) in the following lymph node basins: head and neck (n = 4), axillae (n = 14), and groin (n = 15). Sixteen of 30 lesions (53%) were in patients who had normal findings on clinical examination at entry (stage IB–II) and presented with regional lymph node recurrences within 4–20 mo after 99mTc-MIBI scanning (median, 9 mo). The 3 false-negative cases were in patients with T2 melanomas (1.1–1.4 mm) who developed palpable axillary nodal metastases at 6–18 mo after imaging. 99mTc-MIBI findings were correctly negative for 28 of 33 patients with negative histology findings (n = 2) or a clinically benign course (n = 26).
Anterior image of thorax demonstrates focal area of increased 99mTc-MIBI accumulation in right axilla (arrowhead) in patient with stage II melanoma of trunk. Patient presented with nodal recurrence in same basin 18 mo after scintigraphy. Mask was placed over myocardium.
Anterior pelvic view of patient with stage II melanoma of right leg shows focal tracer uptake in right groin (arrowhead). Nodal recurrence developed in same basin 12 mo after 99mTc-MIBI scanning. Mask was placed over bladder.
A comparison of the results and diagnostic values of 99mTc-MIBI scintigraphy with the final diagnosis (histology and clinical follow-up) is shown in Tables 2 and 3. Differences among the whole population (n = 66) and subsamples with palpable (n = 17) and nonpalpable (n = 49) regional lymph nodes for sensitivity, specificity, LRpos, and LRneg were not statistically significant.
Results of 99mTc-MIBI Scans Compared with Final Diagnosis (Histology/Clinical Follow-up)
Diagnostic Values of 99mTc-MIBI Scintigraphy
DISCUSSION
Studies of a large series of melanoma patients treated with wide local excision of primary tumor showed that the average incidence of recurrence during follow-up was 40%, with regional lymph nodes being the most frequent first site of metastasis (13). Besides, there is no conclusive evidence that laboratory or radiologic follow-up testing of asymptomatic patients with melanomas resected with an intention to cure is more beneficial for detecting early recurrences than is monitoring patients with physical examinations (14).
There is a common misconception that lymphadenectomy offers no therapeutic benefit for melanoma patients because the distinction between elective and therapeutic lymph node dissection is often overlooked or obscured (6). In fact, the available evidence suggests that early therapeutic lymph node dissection for subclinical nodal disease may be superior to delayed lymph node dissection once nodal disease develops (1,15,16). Thus, finding an accurate imaging technique for the detection of early regional lymph node metastases is clinically relevant in melanoma patients.
Clinical evidence shows that lymphoscintigraphy associated with intraoperative γ-probe detection of the sentinel node improves the accuracy of diagnosing lymph node involvement in patients with early-stage melanoma (17). However, the technique is technically challenging, can be applied only through surgery, and is associated with documented complications such as mild to moderate degrees of lymphedema and allergic reactions to isosulfan blue (18,19). Interestingly, nodal recurrences can constitute 28% of first-time recurrences in patients who underwent sentinel node procedures. However, this relatively high rate could be partially related to patients whose sentinel nodes had been incorrectly classified as negative by standard pathology techniques (20).
Initial reports suggest that 99mTc-MIBI scintigraphy is an accurate technique for the detection of recurrent melanoma lesions (11,12,21). In a series with 82 patients studied during postsurgical follow-up, 99mTc-MIBI scanning demonstrated a sensitivity of 0.92 and a specificity of 0.96 for the detection of metastatic lesions (12). However, 35% of patients had clinically or radiologically evident recurrent disease at the time of 99mTc-MIBI imaging. In 14 patients, the procedure detected previously unknown metastatic lesions, including nodal metastases.
In the present study, 99mTc-MIBI scintigraphy resulted in overall sensitivity and specificity of 0.91 and 0.85, respectively, for the detection of regional lymph node metastases in a population with a nodal recurrence prevalence of 50%. The relatively high prevalence observed in patients without palpable regional lymph nodes (39%) can be explained by the high proportion of stage II patients (69%) with Breslow thickness > 2.0 mm, who constitute a well-known high-risk population for regional nodal recurrence (22).
Even though sensitivity was lower for the sample with stage IB–II and specificity was higher than for the sample with stage III (0.84 vs. 100 and 0.87 vs. 067, respectively), the differences were not statistically significant, perhaps because the small number of patients enrolled in the second group (n = 17) could create a relatively high degree of uncertainty in the estimation of diagnostic values. The 3 false-negative results that occurred in this study were in stage IB patients with relatively thin melanomas (1.1–1.4 mm). One study showed that a majority of patients with a Breslow thickness < 2.0 mm had micrometastases only in the sentinel node, whereas with increasing thickness, 2, 3, or more positive nodes were found (23). Therefore, we can presume that these patients had a low tumor burden, probably micrometastases, limited to the sentinel node. Furthermore, the actual false-negative rate for 99mTc-MIBI scintigraphy might have been underestimated in the present study, because a well-documented subset of patients can experience morbidity and mortality from recurrent disease after a 10-y disease-free interval (24,25). However, in nearly 90% of melanoma patients, recurrences develop within the first 2 y after diagnosis (14). Most false-positive results occurred for male patients with abnormal groin tracer uptake with a clinically benign follow-up (n = 4). It has been reported that minimal urinary contamination in male patients may have a random pattern with focal areas of tracer uptake, commonly involving the pelvis (26). Because patients were asked to void shortly before scanning, we can postulate that urine contamination might be one possible cause for those findings. The remaining false-positive case was in a patient with histologically confirmed inflammatory lymph nodes. Previous studies have described 99mTc-MIBI uptake by inflammatory lesions, probably related to the increased blood flow and high metabolic requirements of such tissues (11,27).
From a clinical point of view, 99mTc-MIBI scintigraphy appears to have insufficient sensitivity for the evaluation of subclinical lymph node metastases in patients with clinically localized melanoma, with a false-negative rate of 16%. This fact could be explained by the limited spatial resolution of the gamma camera (9.0 mm). Therefore, the technique cannot replace surgical staging of regional lymph node basins. However, the procedure may possibly have a secondary role in the staging of regional lymph nodes in stage II patients who are not good candidates for sentinel node biopsy. That situation can be found in patients who have had a prior wide local excision, flaps, or grafts that may interfere with lymphatic drainage from the primary lesion, rendering sentinel node biopsy less reliable (17).
18F-FDG PET has proven to be a highly standardized technique that can be applied at any stage of disease management, with an accuracy of more than 90% in the detection of melanoma nodal metastases, including cases with nonpalpable lymph nodes. However, the procedure cannot detect small volumes of subclinical microscopic disease with acceptable sensitivity (7,28). Moreover, the limited availability of cyclotron production and PET scanners in certain regions is a significant disadvantage of the technique. Nevertheless, we cannot conclude that 99mTc-MIBI scanning has better sensitivity than 18F-FDG PET for regional staging of melanoma patients. For that purpose, a clinical trial including a head-to-head comparison of both techniques would be required.
CONCLUSION
99mTc-MIBI is a readily available radiopharmaceutical, and the imaging procedure can be performed using current nuclear equipment. The technique has an acceptable sensitivity and specificity for detecting lymph node metastases in patients with intermediate- to high-risk melanoma. However, even if 99mTc-MIBI can detect nonpalpable nodal metastases, it may not have adequate sensitivity for the diagnosis of subclinical microscopic disease. Therefore, sentinel node biopsy should be the procedure of choice for regional node staging in patients with AJCC stage T2–4 N0 M0 melanoma. 99mTc-MIBI scanning may have a secondary role in patients who are not good candidates for sentinel lymphadenectomy.
Acknowledgments
This work was partially supported by a research grant from the Comisión Honoraria de Lucha Contra el Cáncer, Montevideo, Uruguay, and from the International Atomic Energy Agency, Vienna, Austria.
Footnotes
Received Jan. 16, 2003; revision accepted May 20, 2003.
For correspondence or reprints contact: Omar Alonso, MD, Hospital de Clínicas, Centro de Medicina Nuclear, Av. Italia s/n, Montevideo 11600, Uruguay.
E-mail: oalonso{at}hc.edu.uy