Abstract
Abnormalities of autonomic nervous function are associated with a poor prognosis of patients with chronic heart failure (CHF). We studied the effects of a 6-mo exercise training program on Q–T interval dispersion, heart rate and blood pressure variability, baroreflex sensitivity, myocardial blood flow (MBF), and presynaptic sympathetic innervation in 13 patients with New York Heart Association class II–III heart failure. Methods: MBF was measured with the H215O and C15O technique. Cardiac presynaptic innervation was studied by 11C-hydroxyephedrine (HED) retention assessed with PET. Heart rate and blood pressure variability and baroreflex sensitivity were tested with the phenylephrine method. All studies were performed before and after a 6-mo exercise training program. The exercise capacity was determined by spiroergometry, and Q–T dispersion was measured from a standard 12-lead electrocardiogram. Results: Q–T dispersion was reduced after the training period (mean ± SEM, from 52 ± 5 to 36 ± 5 ms [P = 0.01]). Global 11C-HED retention improved from 0.228 ± 0.099 to 0.263 ± 0.066 s−1 (P < 0.05). Global MBF was not affected by training, but MBF increased in areas of low initial perfusion in patients with coronary artery disease (from 0.382 ± 0.062 to 0.562 ± 0.083 mL/g/min [P < 0.005]). The high-frequency spectrum and total R-R interval variability increased (from 4.53 ± 0.30 to 5.02 ± 0.36 ms2 [P < 0.05] and from 3.60 ± 0.34 to 4.31 ± 0.37 ms2 [P < 0.005], respectively). Both changes correlated significantly with the observed change in 11C-HED retention. There was a significant reduction of total and a near-significant reduction of low-frequency (LF) systolic blood-pressure (SBP) variability (from 4.89 ± 1.03 to 3.18 ± 0.48 [P < 0.05] and from 2.79 ± 0.38 to 1.76 ± 0.24 [P = 0.059], respectively). The decrease in LF SBP variability correlated inversely with the enhancement of 11C-HED retention (r = −0.66; P < 0.05). Baroreflex sensitivity increased from 5.83 ± 0.82 to 10.15 ± 1.66 ms/mm Hg (P < 0.05). Conclusion: Exercise training induces beneficial changes in functional and imaging measures of cardiovascular autonomic nervous control. These observations point to a training-induced shift toward normalization of the compensatory autonomic nervous imbalance in CHF.
- chronic heart failure
- exercise training
- hydroxyephedrine
- autonomic nervous function
- myocardial blood flow
Chronic heart failure (CHF) is accompanied by autonomic nervous imbalance, characterized by reduced parasympathetic activity and enhanced sympathetic activity. Several methods measure this imbalance (i.e., heart rate variability), and these methods can be used to predict the outcome of patients with CHF (1,2). Although sympathetic activity predominates, cardiac sympathetic presynaptic nerve function is disturbed (3,4). The disturbed presynaptic sympathetic function of the heart has also been assessed by 123I-metaiodobenzylguanidine (MIBG) SPECT and shown to be associated with a poor prognosis (5,6). 11C-Hydroxyephedrine (HED) is a false norepinephrine analog that, like MIBG, has the same presynaptic neuronal uptake and storage mechanisms as norepinephrine. Recently, a PET study showed that diminished cardiac uptake of 11C-HED also correlates with the poor outcome of patients with CHF (7).
Exercise training has beneficial effects on different indices of heart rate variability in CHF patients (8). It is not known how this translates to the effects on cardiac sympathetic nervous function, baroreflex sensitivity, or Q–T interval dispersion (9), additional parameters that are partially regulated by the autonomic nervous system and with the prognostic impact on cardiac patients. Therefore, we studied the effects of a 6-mo exercise training protocol on exercise performance, pulmonary oxygen uptake, cardiac presynaptic sympathetic nervous function, Q–T interval dispersion, baroreflex sensitivity, and heart rate and blood pressure variability in 13 patients with CHF. Our hypothesis was that abnormalities of cardiac presynaptic innervation would be at least partially reversible by exercise and that this improvement would be accompanied by beneficial changes in functional measures of the autonomic nervous system.
MATERIALS AND METHODS
Study Group
The study group consisted of 13 patients (12 men, 1 woman; age, 58.2 ± 7.1 y; ejection fraction, 36% ± 5% [mean ± SD]) with stable New York Heart Association class II–III CHF. The etiology of CHF was coronary artery disease in 11 patients and dilated cardiomyopathy in 2 patients. One of the patients had chronic atrial fibrillation, whereas the others had sinus rhythm. Three patients had diabetes. The patients’ permanent medication included angiotensin-converting enzyme inhibitors (n = 10), diuretics (n = 7), nitrates (n = 9), digoxin (n = 4), and β-blockers (n = 8). All coronary patients had had at least 1 myocardial infarction and 10 had undergone either coronary bypass or percutaneous transluminal coronary angioplasty. The time between the last myocardial revascularization procedure and the beginning of the study was 60 ± 57 mo (minimum, 6 mo), and the time between the last myocardial infarction and the beginning of the study was 64 ± 62 mo. The study was performed in accordance with the Declaration of Helsinki and with the approval of the local ethics committee. All patients gave written informed consent to participate in the study.
Exercise Training
All patients participated in a 6-mo exercise training program, which consisted of aerobic and anaerobic training once daily, 6 d/wk. This home-based training period was preceded by a 2-wk in-house instruction period in a rehabilitation center. The exercise protocol has been described in detail (10). No changes in medication were made during the study period.
Baseline measurements were performed and a personal training program was created for each participant during the in-house period. Individualized target heart rate levels below the ischemic threshold were determined using information from a standard stress test and telemetric surveillance of the subjects’ responses during the in-house training. The anaerobic exercise consisted of light-intensity circuit muscle training and included the dorsal and abdominal musculature as well as the muscles of the upper and lower extremities. Depending on each subject’s preference, the aerobic training consisted of walking, step-board exercise, ergometer training, or a similar aerobic exercise method (minimum duration, 30 min). Compliance was assessed at monthly visits and by inspection of the patients’ exercise diaries.
PET
C15O and H215O were produced as described (11).
11C-HED was prepared as described by Någren et al. (12) by allowing 11C-methyl triflate to react with metaraminol. The radiochemical purity exceeded 97%, and the specific radioactivity was 17.5 ± 7.4 GBq/μmol at the time of administration.
The subjects were positioned supine in an 8-ring ECAT 931/08-12 tomograph (Siemens/CTI, Knoxville, TN). C15O and H215O studies were performed as described (11). Immediately after these studies, 686.7 ± 169.5 MBq 11C-HED were injected intravenously over a 60-s period, and 11C-HED imaging was continued for 40 min. All data were corrected for dead time, decay, and photon attenuation, and transaxial images were reconstructed in a 128 × 128 matrix. The final in-plane resolution of the reconstructed and Hann-filtered (0.3 cycle/s) images was 9.5-mm full width at half maximum. The radioactivity of the images was corrected for partial-volume effects using information from echocardiographic measurements of heart wall thickness and phantom studies (13,14). Calculations of mean global myocardial blood flow (MBF) were based on a single-compartment model (15,16). Twenty-five venous blood samples were drawn and centrifuged, and the radioactivity of the supernatant was measured in a well counter to obtain the 11C-HED plasma time-activity curve. Five blood samples at 2, 5, 10, 20, and 40 min after the 11C-HED injection were collected for metabolite analysis (17). A monoexponential correction function was then fitted and used to generate the plasma input function corrected for metabolites.
Large horseshoe-shaped regions of interest (ROIs) were drawn in 6–10 transaxial planes, carefully avoiding the myocardial borders. Septal, anterior, and lateral segments were analyzed regionally by drawing ROIs to each of these segments separately. The 11C-HED retention index (RI) was calculated as:
 This equation yields a value of the RI based on the mean tracer counts within the myocardial ROIs between 30 and 40 min divided by the integrated metabolite-corrected time-plasma radioactivity curve from 0 to 40 min as of the injection. The mean RI of all slices was taken as the measure of 11C-HED retention. In patients who had a previous myocardial infarction, the mean 11C-HED RI was calculated in the noninfarcted and the infarcted myocardium. The area of infarction was determined by echocardiography and electrocardiographic findings. The regional MBF was measured in the same sets of regions. In final analysis, segments with a low resting blood flow (<0.50 mL/min/g) were also analyzed separately.
This equation yields a value of the RI based on the mean tracer counts within the myocardial ROIs between 30 and 40 min divided by the integrated metabolite-corrected time-plasma radioactivity curve from 0 to 40 min as of the injection. The mean RI of all slices was taken as the measure of 11C-HED retention. In patients who had a previous myocardial infarction, the mean 11C-HED RI was calculated in the noninfarcted and the infarcted myocardium. The area of infarction was determined by echocardiography and electrocardiographic findings. The regional MBF was measured in the same sets of regions. In final analysis, segments with a low resting blood flow (<0.50 mL/min/g) were also analyzed separately.
To correct for any blood flow-related variability of tracer delivery, we divided the regional 11C-HED RIs by regional MBF using the formula:
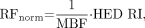 where RFnorm is the 11C-HED RI normalized by MBF. The result is blood flow-corrected 11C-HED retention (18).
where RFnorm is the 11C-HED RI normalized by MBF. The result is blood flow-corrected 11C-HED retention (18).
As a control group for 11C-HED retention, 5 male CHF patients underwent 2 repeated 11C-HED studies in our center. Their left ventricular ejection fraction was 28% ± 7% (age, 47 ± 11 y) with the etiology of CHF being dilated cardiomyopathy in 3 patients and ischemic heart disease with prior myocardial infarctions in 2 patients. The interval between the 2 11C-HED studies was 156 ± 23 d. No flow studies were performed on these subjects. In these sedentary patients, there was no change in 11C-HED retention between the 2 PET studies (11C-HED RI, 0.221 ± 0.028 vs. 0.192 ± 0.057 [P = not significant]).
Exercise Capacity and Cardiopulmonary Performance
Pulmonary oxygen uptake was measured with the EOS-Sprint Exercise Test System (Erich JAEGER GmbH & CoKG, Wurtzburg, Germany) while the subject underwent a symptom-limited exercise test with increments of 15 W/min on an Ergometrics 800S bicycle ergometer (Ergometersysteme GmbH & CoKG, Bitz, Germany).
Autonomic Nervous Function
Electrocardiographic and photoplethysmographic finger arterial pressure signals (Finapres 2300 BP Monitor; Ohmeda Inc., Englewood, CO) were recorded for 5 min at supine rest during controlled breathing at 0.25 Hz. The measurements were repeated in connection with a phenylephrine test at supine rest. A standard IBM personal computer-compatible microcomputer equipped with a software package CAFTS (Medikro Oy, Kuopio, Finland) was used (19). The patients rested for at least 15 min to stabilize their hemodynamics before the study.
A series of >200 R-R intervals free of ectopic beats was chosen from the 5-min recordings. Modified covariance autoregressive modeling with a fixed model order of 14 was used for the heart rate and systolic blood pressure (SBP) variability analysis as described (19). Three spectral bands were used: total power (0.00–0.40 Hz), a low-frequency (LF) band (0.03–0.15 Hz), and a high-frequency (HF) band (0.15–0.40 Hz). The patient with chronic atrial fibrillation was excluded from these tests.
Baroreflex sensitivity was measured after a 150-μg intravenous bolus of phenylephrine by plotting the beat-to-beat values of at least 6 R–R intervals against the SBP values of the preceding cardiac cycle from a user-defined period (20,21). The slope of the linear regression line is the measure of baroreflex sensitivity. The patients with chronic atrial fibrillation were excluded also from baroreflex sensitivity testing.
Q–T Dispersion
The duration of the Q–T intervals was measured from standard 12-lead electrocardiograms recorded on a Marquette 12SL system (sampling frequency, 250 Hz; resolution, 5 μV; Marquette Electronics, Milwaukee, WI) using a paper speed of 50 mm/s. The Q–T intervals were measured manually from the beginning of the QRS complex to the end of the T wave at the point where the terminal limb joined the TP baseline. When U waves were present, the Q–T was measured to the nadir of the curve between the T and U waves. In each recording, the Q–T dispersion was calculated as the difference between the longest and the shortest Q–T interval.
Statistical Analysis
The values before and after the exercise intervention were compared with paired t tests (BMDP Solo; BMDP Statistical Softwares, Inc., Los Angeles, CA). P < 0.05 was considered statistically significant in the 2-tailed tests. All efficacy variables were normally distributed (P > 0.01; D’Agostino test) and they were homoscedastic. The conclusions are thus based on parametric tests. Because of the limited sample size, nonparametric tests (paired Wilcoxon) were also performed. However, because all comparisons with statistical significance were significant using both tests, only results of the Student t test are presented. Linear correlation coefficients (Pearson) were calculated to characterize any associations between the variables. Data are presented as mean ± SE unless stated otherwise.
RESULTS
Exercise Capacity
There was a trend toward improvement in peak pulmonary oxygen uptake (peak VO2) following the exercise program (9% increase from 17.9 ± 1.2 to 19.4 ± 1.7 mL/kg/min [P = 0.13]). The mean workload of the last 4 min of exercise increased from 99 ± 9 to 115 ± 8 W (+16% [P < 0.05]).
MBF
The training program did not affect mean MBF (Table 1). On the other hand, when areas of initially low MBF (<0.50 mL/min/g) were analyzed separately in patients with coronary artery disease (n = 11), MBF increased significantly (0.382 ± 0.017 vs. 0.562 ± 0.025 mL/g/min [P < 0.0005]). Areas with an initial MBF of >0.50 mL/g/min showed no change in MBF (0.808 ± 0.144 vs. 0.781 ± 0.110 [P = not significant]).
Effect of 6-Month Physical Training Program on 11C-HED Retention and MBF
11C-HED Retention
Myocardial 11C-HED retention improved significantly after exercise training (from 0.228 ± 0.027 to 0.262 ± 0.018 s−1 [P < 0.05]). This was true for the 11C-HED RI values and for the 11C-HED retention corrected for regional MBF (Table 1). 11C-HED retention increased most markedly in the lateral segments of the myocardium; indeed, there was no statistically significant change in the anterior wall. Interestingly, there was a trend toward increased 11C-HED retention corrected for blood flow in areas of previous myocardial infarctions (Table 1).
Autonomic Nervous Function
Because of chronic atrial fibrillation in 1 patient, 12 patients were studied. The mean R–R interval measured with the patients supine increased after 6 mo of exercise (from 796 ± 41 to 888 ± 45 ms [P < 0.005]) (Table 2). The total R–R interval variability and the HF band R-R interval variability increased from 4.53 ± 0.30 to 5.02 ± 0.36 ms2 (P < 0.05) and from 3.60 ± 0.34 to 4.31 ± 0.37 ms2 (P < 0.005), respectively. The LF R-R interval variability did not change (Table 2). The enhancement in 11C-HED retention correlated with the increase in total R-R interval variability and with the increase in the R-R interval variability of the HF band (Fig. 1A) (r = 0.55 [P < 0.05] and r = 0.53 [P < 0.05], respectively).
(A) Correlation between change in 11C-HED RI (s−1) and change in HF spectrum of R-R interval (ms2). (B) Correlation between change in 11C-HED RI (s−1) and change in LF spectrum of SBP (mm Hg · ms).
Autonomic Nervous System Function (n = 12) and Q–T Dispersion (n = 13) Before and at End of 6-Month Physical Training Program
Baroreflex sensitivity increased from 5.83 ± 0.82 to 10.15 ± 1.66 ms/mm Hg (P < 0.05).
Regarding SBP variability, the total variability decreased significantly and the LF SBP variability tended to decrease (Table 2). The HF SBP variability was not affected by the 6-mo training program. An inverse correlation was found between the reduction in SBP LF band variability and the increase in 11C-HED retention (Fig. 1B) (r = −0.66 [P < 0.05]).
Q–T Dispersion
The Q–T dispersion was significantly reduced during the study period (Table 1) (from 52 ± 5 to 36 ± 5 ms [P < 0.05]). The change in Q–T dispersion did not correlate with the change in 11C-HED retention.
DISCUSSION
Abnormalities of autonomic nervous function are of prognostic importance in patients with CHF. Like β-receptor antagonists, which were previously considered harmful to CHF patients but are now known to improve survival in this patient group (22–24), exercise training has been transformed similarly to a well-documented adjunctive treatment. Whether exercise training has beneficial effects on survival remains to be shown, but it is known to have positive effects on markers of sympathetic overactivity, such as elevated plasma catecholamine concentrations and reduced heart rate variability (8,25). In this study we show that abnormalities of cardiac presynaptic sympathetic innervation can be partially reversed by exercise and that this improvement is accompanied by beneficial changes in functional measures of autonomic nervous system.
Sympathetic nervous dominance and parasympathetic withdrawal are characteristic findings in CHF. 11C-HED is a false norepinephrine analog that shares the vesicular storage and neuronal uptake mechanisms with norepinephrine (26). Abnormalities in 11C-HED retention have been found in patients with CHF (4), diabetic cardiac neuropathy (27), and cardiomyopathy (28). Whether our observation of improvement in 11C-HED retention is caused by an enhanced uptake-1 mechanism, partial reinnervation or diminished release of 11C-HED from neuronal storage vesicles cannot be deduced from this study.
Because 11C-HED retention is affected by regional blood flow, we also studied the effect of blood flow on 11C-HED retention. Although the mean MBF was unchanged by the exercise program, the MBF did increase in areas of initially low perfusion in patients who had a previous myocardial infarction. Because 11C-HED retention was enhanced also when corrected for regional differences and changes in MBF, there is apparent improvement in cardiac sympathetic presynaptic function.
According to 123I-MIBG studies, partial reinnervation of the periinfarct myocardium takes place during the months after an acute myocardial infarction (29). The situation is apparently similar in our study. We observed a trend toward improvement in the areas of previous myocardial infarction after the 6 mo of training. Because the time between any revascularization procedure or acute coronary event and this study was long, the findings are probably associated with the effects of exercise training and not with any common effects that follow the recovery from a coronary event. This conclusion is supported by the observation that there was no change in the retention of 11C-HED during a respective time period in sedentary CHF patients studied in our center.
Patients with CHF have a high risk of sudden death. Sympathetic overactivity in the absence of parasympathetic stimulation predisposes to ventricular arrhythmias (30). The pathophysiologic significance of reduced 11C-HED retention could be related to increased susceptibility of the denervated but viable myocardium (often seen in infarct and periinfarct zones) to catecholamine stimulus combined with reduced parasympathetic activity. Reduced and heterogeneous 11C-HED retention may also signify the presence of repolarization gradients in the myocardium (3). All of these pathophysiologic mechanisms may contribute to arrhythmogenesis in CHF patients.
Diminished heart rate variability is of prognostic significance in CHF (2). The training program of this study led to expected increases in total and HF R–R interval variabilities. These changes correlated with the change in 11C-HED retention. Also, baroreflex sensitivity improved significantly, as did the total SBP variability, whereas the LF SBP variability tended to improve. Diminished baroreflex sensitivity predicts poor survival of patients with symptomatic CHF (1,31) and of patients with low ejection fraction after myocardial infarction (32). 11C-HED retention correlates with baroreflex sensitivity and LF SBP variability in patients with CHF (4). In this study, we expanded these findings and also observed that changes in LF SBP variability correlate with changes in 11C-HED retention (Fig. 1).
Vasoconstriction is an integral part of the hemodynamic disturbances in CHF. LF SBP variability is believed to be caused primarily by sympathetically mediated fluctuations of vascular tone and peripheral resistance (33–35). Therefore, the decrease in LF SBP variability after training that was induced in this study may reflect a decrease in sympathetic neural traffic to the periphery; this reduced neural input could then lead to decreased peripheral vascular resistance. The correlation between the change in HED retention and reduction of LF SBP variability by training suggests that these 2 measures of sympathetic nervous system may have a common, perhaps a central, regulatory mechanism. The relation may also be fortuitous and there could be separate beneficial effects of exercise on different levels of the sympathetic nervous system. In contrast, HF R–R interval variability reflects preferentially parasympathetic activity; somewhat surprisingly, the change in HF R–R interval variability correlated with the change in 11C-HED retention. Although our data do not provide an explanation for this correlation, abnormalities of the parasympathetic nervous system and the sympathetic nervous system seem to be partially corrected by exercise, and they could result in a shift toward improved parasympathetic-sympathetic balance in CHF patients. It is also noteworthy that no difference in the outcome of the exercise intervention in any studied parameter was observed between the subjects receiving and those not receiving β-adrenoceptor antagonists (data not shown).
Increased Q–T dispersion correlates with a poor prognosis of CHF patients (36). Our findings are in accordance with a previous report (9), according to which a decrease in Q–T dispersion occurs after exercise training, even in the absence of significant improvement of exercise capacity. Our 6-mo exercise training protocol resulted in an improvement of the average workload of the last 4 min of exercise in our patients, but there was no significant change in peak pulmonary oxygen uptake. It is possible that all benefits of physical training do not require equal intensity of training. This may be especially true for the effects on autonomic nervous function because beneficial changes in heart rate variability have been reported in a protocol in which the peak VO2 improvement was only of borderline significance (8).
Exercise training of patients with ischemic cardiomyopathy improves thallium uptake and the change in uptake correlates with coronary collateral score, suggesting improved MBF (37). In our study, only areas with low initial flow had greater MBF after the study period. Whether the improved flow in certain regions is associated with enhanced collateral flow, improved vasodilatory action caused by beneficial changes in endothelial function, or some other mechanism has not been established. Because of the relatively exhausting PET scanning protocol, we were unable to include the determination of myocardial perfusion reserve in our patients with CHF. Therefore, the functional significance of this change in resting coronary flow to tolerance of ischemia is unclear and must be interpreted with caution.
CONCLUSION
Disturbances of the parasympathetic nervous system and the sympathetic nervous system, including cardiac presynaptic innervation as assessed by 11C-HED PET, can be partially reversed by exercise training in CHF patients. The fact that several of these measures are known to have prognostic value provides a theoretic background for the potential survival benefit by exercise training. Finally, because improved 11C-HED uptake correlates with beneficial changes in the function of the autonomic nervous system, the use of presynaptic innervation imaging as a tool to individualize CHF therapy deserves further study.
Acknowledgments
The authors acknowledge Satu Laaksonen, MD; Katariina Vänttinen, MD; Hilkka Sivula, MD; Markku Saraste, MD; and Heikki Ukkonen, MD, for expert technical assistance. The authors thank Robert Paul, MD, for critical review of this manuscript. Financial support was received from the Finnish Heart Foundation and Turku University Central Hospital EVO Fund.
Footnotes
Received Sep. 5, 2001; revision accepted Feb. 12, 2002.
For correspondence or reprints contact: Mikko Pietilä, MD, Department of Medicine, Turku University Central Hospital, Kiinamyllynkatu 4-8, 20520 Turku, Finland.
E-mail: mikko.pietila{at}tyks.fi








