Abstract
PET with 18F-FDG is used for detection and staging of thoracic cancer; however, more specific PET radiopharmaceuticals would be welcome. 11C-labeled choline (CHOL) is a new radiopharmaceutical potentially useful for tumor imaging, since it is incorporated into cell membranes as phosphatidylcholine. The aim of this study was to investigate whether 11C-CHOL PET has advantages over 18F-FDG PET in patients with thoracic cancer. Methods: We evaluated 17 patients with thoracic cancer both with 11C-CHOL PET and 18F-FDG PET. After transmission scanning, 11C-CHOL was injected intravenously, and whole-body scanning was started after 5 min. Immediately thereafter, 18F-FDG was injected intravenously, followed after 90 min by interleaved attenuation-corrected whole-body scanning. Scans were performed from crown to femur. Visual and quantitative (standardized uptake value) analyses of 11C-CHOL PET and 18F-FDG PET were performed and compared with results of traditional staging and follow-up. Results: The most prominent features of normal 11C-CHOL distribution were high uptake in liver, renal cortex, and salivary glands. Except for some uptake in choroid plexus and pituitary gland, brain uptake was negligible. All primary thoracic tumors were detected with 11C-CHOL PET and 18F-FDG PET. Both 11C-CHOL PET and 18F-FDG PET correctly identified all 16 patients with lymph node involvement. However, in a lesion-to-lesion analysis, 11C-CHOL PET detected only 29 of 43 metastatic lymph nodes, whereas 18F-FDG PET detected 41 of 43. 11C-CHOL PET detected fewer intrapulmonary and pleural metastases than 18F-FDG PET (27/47 vs. 46/47). More brain metastases were detected with 11C-CHOL PET (23/23) than with 18F-FDG PET (3/23). For primary tumors, the median (range) standard uptake values of 11C-CHOL and 18F-FDG were 1.68 (0.98–3.22) and 4.22 (1.40–8.26), respectively (P = 0.001). Conclusion: 11C-CHOL PET can be used to visualize thoracic cancers. Although detection of lymph node metastases with 11C-CHOL PET was inferior compared with 18F-FDG PET, the detection of brain metastases was superior.
PET is a noninvasive metabolic imaging technique and a useful clinical tool for staging lung malignancies (1). Most of the experience in lung cancer imaging has been gained with the glucose analog 18F-FDG, a marker of glycolysis (2). In the characterization of malignant solitary pulmonary nodules, 18F-FDG PET yields sensitivities over 90% (3,4). For mediastinal staging in non–small cell lung cancer, PET is superior to CT (1,5). However, 18F-FDG has some disadvantages; for example, uptake in inflammatory tissue may be responsible for false-positive results (6,7). Another problem is a reduction in cellular 18F-FDG uptake in hyperglycemia (8). Also, routine whole-body 18F-FDG PET lacks sensitivity for imaging brain metastases, a common event in lung cancer, because glucose is avidly taken up by the brain.
11C-labeled choline (CHOL) has recently been described as a PET tracer for tumor detection that may not have these drawbacks (9). Choline is one of the components of phosphatidylcholine, an essential element of phospholipids in the cell membrane (10). Malignant tumors may show high proliferation and increased metabolism of cell membrane components, leading to increased uptake of choline (11). There is limited experience with this tracer. One study reported that it was possible to detect several different tumor types with high accuracy (12). Another study suggested that 11C-CHOL PET was more effective than 18F-FDG PET and CT in detecting very small mediastinal metastases in patients with esophageal carcinoma (13). Since normal brain cells are in a nondividing state, it is assumed that uptake of 11C-CHOL by normal brain cells is very low. However, brain tumors and metastases are characterized by increased cell membrane synthesis, enabling them to be visualized with 11C-CHOL PET (14). 11C-CHOL is very rapidly cleared from the blood pool, and optimal tumor-to-background contrast is reached within 5 min, so scans can be started within a few minutes after injection of the radiopharmaceutical (9,13,14). This results in a short protocol with a minimal interval after injection of 11C-CHOL, which is convenient for the patient. In contrast, 18F-FDG scans are routinely started 90 minutes after injection of the radiopharmaceutical to increase tumor-to-background contrast.
Considering the ability of thoracic cancers to metastasize locoregionally and hematogeneously, a whole-body PET scan to stage the disease is appealing. The aim of this study was to determine the feasibility of whole-body 11C-CHOL PET for detection of primary thoracic cancers and their metastases, and to compare this technique to whole-body 18F-FDG PET.
Materials and Methods
Patients
The study population consisted of 17 patients with histologically proven thoracic cancer. Tumor size was determined by measuring the 2 largest perpendicular dimensions on a representative transverse CT slice. The minimum diameter of the primary tumor was 1 cm. Mediastinal and distant metastases were evaluated by invasive procedures such as mediastinoscopy, guided biopsy of suspected distant lesions, or subsequent imaging of growing lesions (CT, MRI, sonography, or 99mTc-diphosphonate bone scintigraphy). Patients were studied before start of treatment with chemotherapy or radiotherapy. Excluded from the study were patients with hyperglycemia before the PET study (serum glucose ≥10 mmol/L (180 mg/dL).
The medical ethics committee of Groningen University Hospital approved the study protocol. All patients gave written informed consent.
Data Acquisition
All studies were performed using an ECAT EXACT HR+ (Siemens/CTI Inc., Knoxville, TN). This camera acquires 63 planes simultaneously over a 15.5-cm field of view. In-plane resolution is approximately 4.3 mm, with an axial resolution of approximately 4.1 mm full width at half maximum (15).
18F-FDG was synthesized according to Hamacher et al. (16) by an automated synthesis module (17). 11C-CHOL was synthesized according to Hara et al. (14) by the reaction of 11C-methyl iodide with dimethylaminoethanol at 100°C for 5 min. Unreacted substrates were removed by evaporation, and 11C-CHOL was further purified using a cation-exchange resin. The resulting product was dissolved in saline. Both radiopharmaceuticals were sterile and their radiochemical purity was ≥95%.
Patients were instructed to fast for at least 6 h before imaging to minimize nonspecific uptake of 18F-FDG and 11C-CHOL in normal tissue (9,18). They were also instructed to drink at least 1 liter of water before imaging to stimulate 18F-FDG excretion from the renal calyces and subsequent voiding. A venous canula was inserted in the forearm for injection of the radiopharmaceuticals. From this canula, a 2-mL blood sample was also drawn to measure serum glucose level.
After positioning the patient in the camera, transmission scanning using a 68Ge/68Ga ring source was performed from crown to femur for 3 min per bed position to correct for attenuation of the photons by the body tissues. This was immediately followed by intravenous injection of 800 MBq 11C-CHOL. After a 5-min interval, whole-body scanning was performed over the same area for 5 min per bed position. 18F-FDG was injected intravenously immediately thereafter (400 MBq in patients with body weight <85 kg; 600 MBq in patients with body weight >85 kg). Ninety minutes after 18F-FDG injection (130 min after 11C-CHOL administration), interleaved attenuation-corrected whole-body scanning was performed from crown to femur with 3 and 5 min per bed position for transmission and emission scanning, respectively. Data from multiple bed positions were iteratively reconstructed (ordered subset expectation maximization) into attenuated and nonattenuated 11C-CHOL and 18F-FDG whole-body PET images (19).
Data Analysis
Non–attenuation-corrected 11C-CHOL PET and 18F-FDG PET images were qualitatively compared for their uptake in malignant lesions and normal anatomical structures by 2 experienced PET physicians unaware of patients’ clinical data. If they could not reach consensus, the opinion of a third independent observer was sought. Because detection or exclusion of malignant lesions rather than determination of quantitative parameters was the main goal of this PET study, only non–attenuation-corrected PET images were used for qualitative analysis. The physicians interpreted any hot spots as either benign or malignant.
To compare the results of 11C-CHOL PET, 18F-FDG PET, and histological data, mediastinal hot spots were located according to the Mountain and Dresler classification of regional lymph nodes (20). Hot spots outside the mediastinum were described according to anatomical location.
Standardized uptake value (SUV) was calculated for malignant primary lesions from regions of interest (ROI) obtained from the attenuation-corrected images. SUV provided a measure of tracer concentration in the ROI relative to its uniform distribution over the body. SUV depends, among other things, on patient habitus; to minimize this source of variability, a correction of SUV was made for lean body mass (21). Using ECAT/CAPP software (Siemens/CTI Inc.), SUVs were calculated in the transaxial plane in which the tumor had the highest activity. By using the isocontour tool adjusted to 70% of the peak counts in the lesion, an ROI was drawn from the part of the tumor with the highest activity in a standardized manner.
Statistical Analysis
The degree of interobserver agreement was quantified with the κ statistic. To compare 11C-CHOL and 18F-FDG SUVs of primary lung tumors, Wilcoxon’s signed rank test was used. The correlation coefficient between 18F-FDG and 11C-CHOL SUVs was calculated using 1-tailed Pearson’s test. A probability of ≤0.05 was considered statistically significant. Statistical analysis was performed with SPSS software (Statistical Product and Service Solutions Inc., Chicago, IL).
Results
Patients
A total of 17 patients were included in this study; their characteristics are shown in Table 1. Tumor diameter varied between 2 and 8.5 cm. All 17 patients received 800 MBq 11C-CHOL according to the protocol; 14 patients received 400 MBq 18F-FDG, and the other 3 patients received 600 MBq 18F-FDG.
Patient Characteristics
Physiological Body Distribution of 11C-CHOL
11C-CHOL PET produced easily interpretable images. The most prominent physiological uptake of tracer was observed in the liver, renal cortex, and salivary glands. Less intense uniform tracer uptake was present in the lungs, spleen, skeletal muscles, and bone marrow. Variable 11C-CHOL uptake was observed in the pancreatic region, and a linear uptake configuration in the abdomen was identified as small intestine. In 2 patients, mild uptake of 11C-CHOL was observed in the thyroid gland. Uptake of tracer in the brain was negligible; however, mild tracer uptake was observed in the choroid plexus and pituitary gland. No uptake was observed in the mediastinum and myocardium. A clear distinction could be made between the mediastinum and the lungs. In 2 patients, accumulation of 11C-CHOL in the bladder was observed.
The whole-body distribution of 18F-FDG has been described previously (22). A similar pattern was observed in this study.
Detection of Lesions with 11C-CHOL PET and 18F-FDG PET
Interobserver Agreement. With respect to hot spot detection from 11C-CHOL PET images, the degree of interobserver agreement (κ) was 0.93 (95% confidence interval [CI]; range, 0.87–0.99). For 18F-FDG PET images, agreement was 0.96 (95% CI; range, 0.91–1.00). Visually, 11C-CHOL uptake in malignant lesions often appeared remarkably less intense than that of 18F-FDG.
Detection of Primary Thoracic Tumors. Both 11C-CHOL PET and 18F-FDG PET detected all 17 primary thoracic tumors (Table 2). In 4 patients the 11C-CHOL hot spot appeared as a circular lesion with a rim of mild uptake and a central large defect. The matching 18F-FDG hot spots appeared as circular lesions with intense increased uptake and central localized small defects.
Lesions Detected with 11C-CHOL PET and 18F-FDG PET Compared with Traditional Staging and Follow-Up*>
Detection of Lymphatic Metastases. Ten patients were diagnosed with mediastinal lymph node metastases. In all these patients, both 11C-CHOL PET and 18F-FDG PET detected mediastinal hot spots (Table 2). With surgical procedures, a total of 33 metastatic mediastinal lymph node stations were confirmed; 18F-FDG PET detected 31 of the 33 (94%), while 11C-CHOL PET detected only 21 of 33 (64%) (Fig. 1). Both 11C-CHOL PET and 18F-FDG PET were false-positive at 1 mediastinal lymph node station in the same patient. At another mediastinal lymph node station, 11C-CHOL PET, but not 18F-FDG PET, was false-positive.
Consecutive coronal planes of non–attenuation-corrected 18F-FDG PET (upper row) and 11C-CHOL PET (lower row) images. 18F-FDG images show intense uptake in primary tumor, hilar lymph node metastasis, and mediastinal lymph node metastases. 11C-CHOL-PET images show mild tracer uptake in primary tumor and hilar metastasis; the mediastinal lymph node metastases are missed. Note high uptake of 11C-CHOL-PET in liver and kidneys, hampering interpretation of upper abdomen.
Both 11C-CHOL PET and 18F-FDG PET were correct in detecting all 6 patients with supraclavicular or axillar lymph node metastases. A total of 10 cytologically proven supraclavicular or axillar lymph node metastases were found, of which 11C-CHOL PET detected 8 (80%) while 18F-FDG PET detected all (100%). At 1 supraclavicular lymph node site, both 11C-CHOL PET and 18F-FDG PET were false-positive.
Detection of Hematogenic Metastases. Both 11C-CHOL PET and 18F-FDG PET correctly detected all 7 patients with intrapulmonary or pleural metastases (range, 1–23 metastases) (Table 2). In 1 patient, both 11C-CHOL PET and 18F-FDG PET were false-positive for 1 intrapulmonary hot spot. With 11C-CHOL PET, hot spots were observed at 27 of 47 (57%) intrapulmonary or pleural metastatic sites; with 18F-FDG PET, hot spots were observed at 46 of 47 (98%) sites.
In 1 patient, bone scintigraphy revealed 4 bone metastases; both 11C-CHOL PET and 18F-FDG PET detected skeletal hot spots at the same locations. In a second patient, 11C-CHOL PET revealed hot spots in the right humerus and right pelvic bone, while 18F-FDG PET only revealed a hot spot in the right humerus. Bone scintigraphy revealed no suspicious skeletal lesions at all.
An adrenal gland metastasis in 1 patient and 2 liver metastases in another patient were confirmed; these were correctly diagnosed by 18F-FDG PET but not detected by 11C-CHOL PET. In 1 patient, both 11C-CHOL PET and 18F-FDG PET were false-positive for a hot spot ranked as an adrenal gland metastasis.
CT or MRI detected 23 brain metastases in 3 patients. 11C-CHOL PET was able to detect all brain metastases (100%), whereas 18F-FDG PET only detected 3 (13%).
SUV Analysis
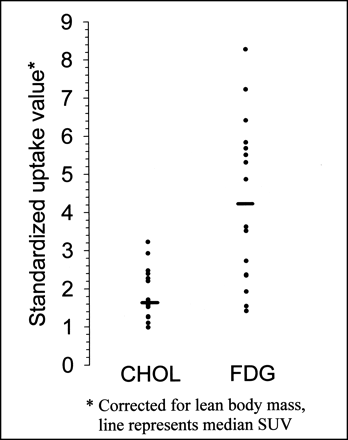
SUVs (corrected for lean body mass) of the primary lung tumor are presented in Figure 2. Apart from 1 patient, SUVs were higher for 18F-FDG than for 11C-CHOL. The median (range) of SUVs were 1.68 (0.98–3.22) for 11C-CHOL and 4.22 (1.40–8.26) for 18F-FDG (P = 0.001). The 11C-CHOL SUV and 18F-FDG SUV of primary tumors were only weakly correlated (correlation coefficient, 0.47; P = 0.03).
PET SUVs of 11C-CHOL and 18F-FDG of primary tumors in patients with thoracic cancer.
Discussion
The most widely applied PET radiopharmaceutical in oncology is 18F-FDG. Although 18F-FDG PET studies have additional value in detection, staging, and treatment monitoring in a variety of neoplasms (23), 18F-FDG PET is not 100% accurate in the detection of primary tumors and their metastases (24). Therefore, more specific PET radiopharmaceuticals are needed.
Choline is transported into cells and acts as a precursor for the biosynthesis of phospholipids, which are membrane elements (10). Cancer is associated with cell proliferation and upregulation of the enzyme choline kinase (which catalyzes the phosphorylation of choline in the pathway for biosynthesis of phospholipids) (25), providing the rationale for the use of 11C-CHOL as a radiopharmaceutical in oncological PET studies. In the brain and prostate, organs in which 18F-FDG PET lacks sensitivity, 11C-CHOL PET showed considerable potential for the detection and staging of tumors (14,26).
PET tumor detection depends on the tumor-to-nontumor uptake ratio. Although background uptake of 11C-CHOL and 18F-FDG in the lung was uniform, 11C-CHOL uptake in malignant lesions was lower compared with 18F-FDG. The uptake of 11C-CHOL in tumors represents membrane synthesis, whereas 18F-FDG uptake represents glycolysis. However, 18F-FDG is also taken up by inflammatory cells such as macrophages (6). Large cavitating lung tumors may be associated with a rim of inflammatory cells with elevated 18F-FDG uptake surrounding a necrotic tumor region. Tumor hypoxia also affects cellular tracer uptake. Tumor hypoxia increases cellular uptake of 18F-FDG, while the uptake of other substrates (e.g., amino acids) is decreased (27). Similarly, we observed reduced uptake of 11C-CHOL in tumor areas that were presumed to be hypoxic.
The occurrence of locoregional or distant metastases has a profound effect on the survival of patients with thoracic cancer. Normal tracer accumulation in various organs hampers the detection of metastases with PET in these organs. In the case of 11C-CHOL, free intracellular choline is not only phosphorylated, but it can also be oxidized to betaine aldehyde (10). Since the liver and kidney are major sites for choline oxidation, they exhibit a high background uptake (26,28). For this reason, liver and adrenal gland metastases were not detected by 11C-CHOL PET nearly as well as by 18F-FDG PET. The enhanced uptake of 11C-CHOL observed in the pancreas and intestine of several patients may be due to secretion of phospholipid-rich pancreatic juice and bile (9,26,29).
Consistent with previously reported data (14,30), 11C-CHOL PET was very effective in detecting brain metastases. 11C-CHOL penetrates the blood–brain barrier by the amine-specific transport system (31), followed by a rapid brain washout (28). Disruption of the blood–brain barrier is observed in brain tumors and metastases, and this may facilitate increased 11C-CHOL uptake for cell membrane synthesis. Incorporation of 11C-CHOL in the endothelium of cerebral blood vessels present in the choroid plexus and pituitary gland (32), which do not have a blood–brain barrier, may explain the increased uptake at these sites. Because of the excessive uptake of glucose in the brain, a routine whole-body 18F-FDG PET lacks the sensitivity to detect brain metastases. Other 11C-labeled radiopharmaceuticals used for the evaluation of brain tumors include 11C-methionine and 11C-tyrosine (33,34). In the detection of brain tumors, 11C-CHOL may be preferred to amino acids due its higher tumor-to-background ratio (14). The detection of bone (marrow) metastases with 11C-CHOL PET and 18F-FDG PET in this study was consistent with the literature (26,35,36). In 2 patients 11C-CHOL uptake in the urinary bladder was observed. This urinary accumulation of 11C-CHOL may be the result of incomplete tubular reabsorption of intact tracer or enhanced excretion of labeled oxidative metabolites (9), as was also suggested by DeGrado et al. (37), who saw accumulation in the bladder while using 18F-labeled choline.
11C-CHOL PET and 18F-FDG PET were equally accurate in detecting lymph node metastases. However, on a lesion-to-lesion basis, 11C-CHOL PET was less sensitive than 18F-FDG PET. Our observations contrast with those of Hara et al. (38), who observed 100% sensitivity for 11C-CHOL PET and 75% sensitivity for 18F-FDG PET in detecting mediastinal lymph node metastases originating from non–small cell lung cancer. This may be partly explained by the low SUV threshold used by Hara et al., as they defined a 40% difference from the SUV of the primary tumor as positive for mediastinal lymph node metastasis. This will easily lead to a high sensitivity for 11C-CHOL PET. Their low sensitivity of 18F-FDG PET could be explained by the short interval of 40 min between the injection of 18F-FDG and the onset of scanning. Tumor concentrations of 18F-FDG do not reach a plateau until 90 min after intravenous injection (39).
Consistent with previous reports, 11C-CHOL PET gave clear images with good contrast at 5 min after injection (26,35). After intravenous injection, tissue uptake and blood clearance is rapid, and the tissue-to-background ratio remains essentially constant over 30 min (9,35). Although the short half-life of 11C-labeled radiopharmaceuticals could limit the applicability of whole-body scanning, we found that PET with 800 MBq 11C-CHOL was feasible in practice to obtain images from crown to femur in thoracic cancer patients. According to our acquisition protocol, the 18F-FDG scan was started 130 min after the 11C-CHOL injection, which is more than 6 half-lives of 11C. It can be assumed that 18F-FDG scanning is not contaminated by residual 11C radioactivity. Whole-body PET to stage thoracic cancer is appealing considering the ability of this disease to metastasize locoregionally and hematogeneously.
Conclusion
Visual analysis of whole-body 11C-CHOL PET was less accurate than that of whole-body 18F-FDG PET for the detection of metastases in thoracic cancer patients due to the lower accumulation of 11C-CHOL in malignant tissue. The inferiority of 11C-CHOL PET was most notable in the detection of lymph node metastases. However, for the detection of brain metastases, 11C-CHOL PET was superior to 18F-FDG PET.
Footnotes
Received May 1, 2001; revision accepted Oct. 24, 2001.
For correspondence or reprints contact: Harry J.M. Groen, MD, Department of Pulmonary Diseases, Groningen University Hospital, Hanzeplein 1, P.O. Box 30.001, 9700 RB Groningen, The Netherlands.
E-mail: h.j.m.groen{at}int.azg.nl