Abstract
186Re-labeled chimeric monoclonal antibody U36 (cMAb U36) was recently evaluated in a phase I dose escalation study in head and neck cancer patients. All 13 patients received 99mTc-labeled cMAb U36 before 186Re-cMAb U36 radioimmunotherapy. The aim of this study was to evaluate the suitability of multiple or limited blood sampling to predict clearance, red marrow absorbed dose, and myelotoxicity of 186Re-cMAb U36. Methods: Population pharmacokinetics of 186Re-cMAb U36 were analyzed with a nonparametric expectation algorithm (NPEM 2) and used for Bayesian analysis of individual patient data to predict cMAb U36 clearance. Results: 186Re-cMAb U36 clearance was most accurately predicted (r = 0.91, P < 0.001) with limited sampling for sample points 4 and 72 h after administration of 186Re-cMAb U36. These predictions were less accurate with 99mTc-cMAb U36 (r = 0.51, P = 0.078 for multiple sampling; r = 0.47, P = 0.104 for sampling at 4 and 21 h after administration). Thrombocytopenia was found to be correlated with the red marrow absorbed dose and was equally well predicted by limited blood sampling after administration of 99mTc-cMAb U36 (r = 0.81, P < 0.01) or 186Re-cMAb U36 (r = 0.79, P < 0.01). Conclusion: Limited sampling seems useful to predict pharmacokinetics and myelotoxicity of 186Re-cMAb U36.
Radiogenic damage to the red marrow is the dose-limiting toxicity in most of the radioimmunotherapy (RIT) studies conducted thus far, resulting in thrombocytopenia and leukocytopenia with a nadir at 4–6 wk after therapy. The red marrow absorbed dose is often found to be related to the severity of myelotoxicity (1). In a previous article (2) we reported on a phase I RIT trial in which 99mTc-labeled chimeric monoclonal antibody U36 (cMAb U36) was administered 1 wk before 186Re-cMAb U36 to head and neck cancer patients. With these radioimmunoconjugates, an intrapatient consistency of pharmacokinetics was shown for the decay-corrected areas under the curve (AUCs) up to 25 h after administration. Because of the short half-life of 99mTc in comparison with 186Re, an accurate estimation of the total AUC based on individual patient data was not possible. In this study, a population-based model was used to compare the data on 99mTc-cMAb U36 and 186Re-cMAb U36 pharmacokinetics obtained from the same group of patients. Subsequently, the potential of limited blood sampling to predict the pharmacokinetics of 186Re-cMAb U36 and the severity of myelotoxicity after RIT was evaluated.
Materials and Methods
Patients and Study Design
The study was approved by the Institutional Review Board of the Vrije Universiteit Medical Center (Amsterdam, The Netherlands) and informed consent was obtained. Study design and methods including antibody production, radiolabeling, safety, imaging, and efficacy data of the trial have been reported previously (2). Before RIT, patients received 740 MBq 99mTc-labeled cMAb U36 (2 mg), followed 1 wk later by a single dose of 186Re-cMAb U36 (12 or 52 mg) in radiation dose–escalating steps of 0.4, 1.0, and 1.5 GBq/m2. Blood samples were collected at 5, 10, and 30 min, and at 1, 2, 4, 16, and 21 h after injection in both studies, as well as at 72 h in the RIT study. Hematologic parameters were obtained on a weekly basis for at least 6 wk, or until recovery of myelotoxicity was observed.
Pharmacokinetic Modeling, Red Marrow Dosimetry, and Myelotoxicity
Blood samples were counted in a multiwell γ-counter (1470 Wizard; Wallac, Turku, Finland) and compared with an aliquot retained from the conjugate preparation for injection. These results, together with patient age, sex, weight, body surface area, and serum creatinine, were analyzed with a nonparametric expectation maximization algorithm (NPEM 2, USC-Pack; Laboratory of Applied Pharmacokinetics, USC School of Medicine, Los Angeles, CA) to reveal the following mean pharmacokinetic parameters: initial volume of distribution (Vd), elimination constant rate (kelm), and transfer rate constants (k12 and k21) (3). The individual pharmacokinetic parameters were calculated with MW/Pharm (Mediware, Groningen, The Netherlands) using the population pharmacokinetic parameters as Bayesian forecast. Subsequently, the following analyses were performed:
Bayesian clearance estimates of cMAb U36 were assessed, using blood activity concentration data of either 99mTc-cMAb U36 or 186Re-cMAb U36. For these estimates data of either all blood samples (“multiple sampling”) or combinations of 2 blood samples (“limited blood sampling”) were used.
The obtained Bayesian clearance estimates were used to calculate the effective clearance according to the following equation: Cleffective = Clbiologic + Clphys, where Cleffective and Clbiologic are the effective and biologic clearance of the radioimmunoconjugate, respectively, and Clphys is the physical clearance of the radionuclide (Vd × ln 2/[half-life of 186Re]; half-life of 186Re = 90.62 h).
The actual effective clearance (i.e., non-Bayesian clearance) was assessed by fitting blood activity concentration data of all blood samples of an individual patient obtained after administration of 186Re-cMAb U36.
Bayesian clearance estimates were compared with the actual non-Bayesian clearance.
To calculate the total AUC in blood (AUCblood), and subsequently the red marrow absorbed dose, the following equation was used:
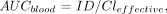 where ID is the total injected therapy dose. This AUCblood, together with the total body radioactivity, was used for calculation of a patient-specific red marrow dose according to the method of Shen et al. (4). The red marrow absorbed dose estimates were compared with the development of myelotoxicity, that is, the nadir and the percentage decrease from baseline values of platelets, white blood cell count, and granulocytes.
where ID is the total injected therapy dose. This AUCblood, together with the total body radioactivity, was used for calculation of a patient-specific red marrow dose according to the method of Shen et al. (4). The red marrow absorbed dose estimates were compared with the development of myelotoxicity, that is, the nadir and the percentage decrease from baseline values of platelets, white blood cell count, and granulocytes.
Statistical Analysis
All mean values reported represent arithmetic means with corresponding SDs. Associations between variables were calculated with SPSS 7.5 software (SPSS Inc., Chicago, IL) using Pearson correlation tests with P < 0.05 considered statistically significant. Multiple regression analysis was performed to find a possible relationship between the covariates age, weight, body surface area, serum creatinine, injected dose, clearance, and red marrow dose in predicting myelotoxicity as determined by the nadir of platelets.
Results
Patient characteristics are shown in Table 1. The mean population parameters obtained with NPEM 2 were kelm = 0.013 h−1 (±0.006), k12 = 0.024 h−1 (±0.018), k21 = 0.215 h−1 (±0.146), and Vd = 0.074 L/kg (±0.011). A mean cMAb U36 clearance of 0.056 L/h (range, 0.036–0.074 L/h) was found in the therapy study with 186Re-cMAb U36 using non-Bayesian analysis. The goodness of fit of the Bayesian and non-Bayesian 186Re-cMAb U36 clearance was assessed (r = 0.99, P < 0.0001).
Patient Characteristics
Bayesian analysis using data from all sample points in the 99mTc-cMAb U36 study was not able to predict with statistical significance the actual 186Re-cMAb U36 clearance for an individual patient (r = 0.51, P = 0.078). The optimal 99mTc-cMAb U36 sample pair for prediction was 4 and 21 h after administration (r = 0.47, P = 0.104).
The combination of sample points at 4 and 72 h after administration of 186Re-cMAb U36 appeared to be the optimal sample pair for prediction of the actual clearance of 186Re-cMAb U36 (r = 0.91, P < 0.001).
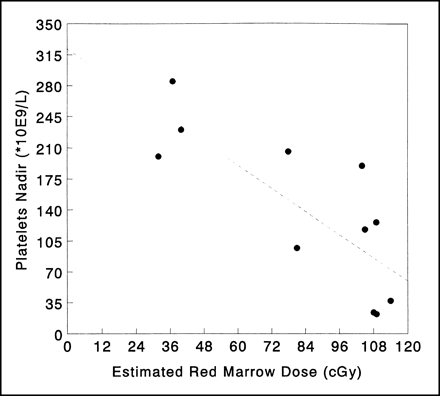
Development of hematologic toxicity could be evaluated for 11 of 13 patients, because 2 patients did not complete the follow-up for evaluation of myelotoxicity. Correlations between myelotoxicity and red marrow absorbed dose estimates are given in Table 2. The most severe toxicity, that is, thrombocytopenia, was shown to be correlated with the red marrow dose calculated from the actual 186Re-cMAb U36 clearance (r = 0.80, P < 0.01, and r = 0.66, P < 0.05, for platelet nadir and percentage decrease, respectively). If the red marrow dose was calculated from the Bayesian estimate of 186Re-cMAb U36 clearance obtained from the 99mTc-cMAb U36 study and normalized with respect to the injected dose of 186Re-cMAb U36 RIT, these correlations appeared to be similar. The relationship between the predicted red marrow doses and development of thrombocytopenia is illustrated in Figure 1.
Relationship between red marrow absorbed dose estimates and development of thrombocytopenia after 186Re-cMAb U36 radioimmunotherapy. Red marrow absorbed dose estimate was calculated using Bayesian analysis of limited blood sampling at 4 and 72 h after administration of 186Re-cMAb U36.
Pearson Correlation Tests for the Prediction of Myelotoxicity
Multiple regression analysis did not show any significant influence of the covariates age, weight, body surface area, or serum creatinine on 186Re-cMAb U36 clearance. The Pearson correlation coefficients for platelet nadir and the different red marrow dose estimates using multiple and limited sampling were all higher than for total injected dose (Table 2). The individual red marrow dose estimate was thus a better predictor of myelotoxicity than total injected dose.
Discussion
The red marrow absorbed dose, determined from the blood time–activity curve, is often a good predictor of myelotoxicity (1). For patient-specific dose planning, accurate red marrow absorbed dose estimation might require frequent sampling, which is a burden for patients, especially when performed in an outpatient setting. In this study, prediction of 186Re-cMAb U36 clearance proved to be feasible when only 2 instead of all blood samples were selected for Bayesian analysis. This prediction was optimal for the sample pair at 4 and 72 h after administration of 186Re-cMAb U36. All patients underwent a preceding scouting study with 99mTc-cMAb U36, but, unfortunately, the prediction of 186Re-cMAb U36 clearance from this scouting study using Bayesian analysis of multiple or limited blood samples was less accurate.
Pharmacokinetics of 99mTc-cMAb U36 and 186Re-cMAb U36 could be different as a result of development of human antichimeric antibody (HACA) responses, which were found in 5 of 13 patients (2), of which 3 had an onset in the week between the 99mTc- and 186Re-cMAb U36 administrations. Because HACA titers were low (<1.50 mg/L) and the amount of administered 186Re-cMAb U36 relatively high (50 mg), the effect of HACA on 186Re-cMAb U36 pharmacokinetics, however, is expected to be small. Presence of antigen at nontumor sites and different antibody doses used for scouting and therapy studies (as was the case in this study) can also explain variation in pharmacokinetics. Previous biodistribution studies with MAb U36, however, showed consistency of pharmacokinetics irrespective of whether the antibody was administered at a dose of 2, 12, or 52 mg (5).
Another explanation for the different pharmacokinetics might be that the short half-life of 99mTc makes the scouting procedure with 99mTc-cMAb U36 of limited value. The short half-life of 99mTc does not allow sampling later than approximately 25 h after administration. Therefore, 99mTc-cMAb U36 might allow accurate prediction of the early part of the 186Re-cMAb U36 clearance but not of the late part. Indeed, biologic AUCs for 99mTc- and 186Re-cMAb U36, as determined up to just 25 h after injection, showed a very strong correlation (r = 0.94, P < 0.01), confirming intrapatient consistency of pharmacokinetics.
Taking the aforementioned information into account we hypothesized that 186Re-cMAb U36 might be a better candidate for such a scouting procedure. 186Re allows sampling at later time points, resulting in a more reliable prediction of cMAb U36 clearance, which was confirmed in this study. 186Re-cMAb U36 clearance was accurately predicted (r = 0.91, P < 0.001) with limited sampling at 4 and 72 h after administration. However, these sampling points were selected from the therapy data, and not from a preceding diagnostic study. Whether the same accuracy can be found if the scouting study is performed with 186Re-cMAb U36 has yet to be shown.
Conclusion
This study shows that limited sampling during a scouting procedure is a realistic option to predict the 186Re-cMAb U36 clearance during RIT. Development of myelotoxicity after 186Re-cMAb U36 RIT correlated well with the derived red marrow dose estimates. 186Re-cMAb U36 seems to be better qualified for such a scouting study procedure than 99mTc-cMAb U36, because of its matched half-life.
Acknowledgments
This study was supported by Dutch Cancer Society grant number VU 96-1313 and by Centocor Inc. (Malvern, PA).
Footnotes
Received Nov. 14, 2000; revision accepted May 14, 2001.
For correspondence or reprints contact: Guus A.M.S. van Dongen, PhD, Department of Otolaryngology/Head and Neck Surgery, Vrije Universiteit Medical Center, De Boelelaan 1117, P.O. Box 7057, 1007 MB Amsterdam, The Netherlands.