Abstract
Radiometal-labeled monoclonal antibodies are retained longer in tumors than iodinated antibodies, leading to their increased use for radioimmunotherapy. Dissociation of radioiodine from the antibody during metabolism has been documented. We now report metabolites in the plasma of lymphoma patients given 111In- and 90Y-2-iminothiolane-2-[p-(bromoacetamido)benzyl]-1,4,7,10-tetraazacyclododecane-N,N′,N″,N‴-tetraacetic acid-Lym-1 (111In/90Y-2IT-BAD-Lym-1). Methods: Nineteen patients with non-Hodgkin’s lymphoma (NHL) received 111In- and 90Y-2IT-BAD-Lym-1; 111In was used as a surrogate tracer for 90Y, which emits no γ-photon. Plasma was obtained up to 7 d and analyzed by high-performance liquid chromatography to determine the fraction of radiolabel associated with monomeric antibody, metabolites, and complexed antibody. Planar images of conjugate views were acquired up to 7 d and used to quantitate 111In in organs and tumors. Results: Metabolites and complexes were observed in the plasma of every patient who received 111In-2IT-BAD-Lym-1. At 3 d, the mean percentages of 111In in the patients’ plasma in monomeric, metabolite, and complexed forms were 54%, 36%, and 10%, respectively. Metabolites of 90Y-2IT-BAD-Lym-1 were formed to a similar extent. In comparison, in groups of breast and prostate cancer patients who received the radioimmunoconjugate 111In-2IT-BAD-m170, 91% and 94% of 111In in the patients’ plasma were in monomeric form, respectively. Metabolites and complexes of 111In-2IT-BAD-Lym-1 contributed a mean 10% of the total area under the time–activity curve (AUC) for blood. Little formation of metabolites and complexes occurred in vitro in NHL patient or volunteer plasma or in Raji cell culture. The clinical and in vitro data supported the processing of 111In/90Y-2IT-BAD-Lym-1 in the hepatocytes as the dominant mechanism for the production of metabolites. Conclusion: Metabolites of 111In/90Y-2IT-BAD-Lym-1 accounted for 10% of blood AUC in patients. The therapeutic index was adversely affected by metabolism of 111In/90Y-2IT-BAD-Lym-1 to the extent that the tumor specificity of the radioactive metabolites was lost.
The area under the curve (AUC) for the blood concentrations of a drug is commonly used to characterize its pharmacokinetic behavior in patients. In the case of radioimmunotherapy using a monoclonal antibody (mAb) labeled with a radionuclide tracer under appropriate conditions, the AUC can be determined by counting sequential blood samples. To the extent that the radionuclide tracks the antibody, the pharmacokinetics of the radiolabeled antibody can be determined for all tissues of the body in a noninvasive manner using quantitative scintigraphy, a remarkable asset. Typically, the stability of the radiolabeled antibody over time in a biologic milieu, such as serum or plasma, is established in preclinical studies (1,2). However, for radioiodinated antibodies the radiolabel is dissociated from the antibody during metabolism (3–6). In such instances, the pharmacokinetic behavior reflects, in part, that of the radionuclide rather than that of the antibody. In any event, the resulting dosimetric determinations are accurate because it is the pharmacokinetics of the radionuclide that determines radiation dose distribution.
Lym-1 has had little therapeutic effectiveness in non-Hodgkin’s lymphoma (NHL) when used for immunotherapy (7), but it has proven highly effective for NHL and B-cell chronic lymphocytic leukemia when used to target radionuclides to malignant tissue (8,9). All histologic grades of chemotherapy-resistant NHL were responsive to 131I-Lym-1 or 67Cu-2-iminothiolane-6-[p-(bromoacetamido)benzyl]-1,4,7,11-tetraazacyclotetradecane-N,N′,N″,N‴-tetraacetic acid-Lym-1 (67Cu-2IT-BAT-Lym-1) (10,11). Radiometals, delivered by antibody carrier molecules, are retained in tumors longer than 131I (albeit also in normal tissues, particularly liver), leading to their increased use for radioimmunotherapy. 90Y was attractive for radioimmunotherapy because it emits abundant, highly energetic β-emissions that deliver a higher radiation dose per unit of radioactivity than 131I or 67Cu (12) and is commercially available. We have used the anti-mouse lymphoma IgG2a Lym-1, radiolabeled as 90Y-2-iminothiolane-2-[p-(bromoacetamido)benzyl]-1,4,7,10-tetraazacyclododecane-N,N′,N″,N‴-tetraacetic acid-Lym-1 (90Y-2IT-BAD-Lym-1), for therapy of NHL (13). 111In was used as a surrogate tracer for 90Y, which lacks a γ-photon emission. 1,4,7,10-Tetraazacyclododecane-N,N′,N″,N‴-tetraacetic acid (DOTA) was designed specifically to bind 90Y tightly, and 90Y- and 111In-2IT-BAD-Lym-1 radioimmunoconjugates (RICs) of high purity, exceptional stability, and complete retention of functional and structural integrity are routinely produced (1,13,14). Molecular sieving high-performance liquid chromatography (HPLC) of plasma and urine samples in phase I trials has been used to confirm that the radionuclide in the plasma remained attached to a species having a molecular weight (MW) corresponding to an mAb, and not lower (metabolites) or higher (complexes) MWs (15,16). HPLC thus served as a simple surrogate for assays of immunoreactivity.
Soon after injection of 111In- or 90Y-2IT-BAD-Lym-1, radiolabeled metabolites and complexes were observed in the plasma of every patient by routine HPLC evaluation. Metabolites of iodinated mAbs, including Lym-1, are routinely identified in patient urine, and to a much lesser extent in the stool (8), but do not commonly accumulate in the plasma. Similarly, metabolites of radiometal-labeled mAbs have been reported in urine of patients (17) but not, to our knowledge, in plasma. In this study, the nature of the metabolites, their impact on therapeutic indices (TIs), and the mechanisms of formation were investigated. The results were compared with those for an antiadenocarcinoma antibody labeled in an analogous fashion and given to patients with breast or prostate cancer.
MATERIALS AND METHODS
Patient Population
Nineteen patients with B-lymphocyte NHL that had progressed despite standard therapy entered studies of 111In-2IT-BAD-Lym-1. The mean age was 53 y (range, 33–77 y). NHL was classified according to the Working Formulation (18), and all grades of lymphoma were eligible for study entry. The mean Karnofsky performance status was 82 (range, 60–90). Lym-1 reactivity was shown in all patients by immunohistologic staining or positive tumor imaging. Two patients had prior splenectomies.
The plasma of all 19 patients who received 111In-2IT-BAD-Lym-1 was examined by radioanalytic HPLC, as described below, for metabolites and complexes. The plasma of 3 of these patients, who subsequently received 90Y-2IT-BAD-Lym-1, was examined by radioanalytic HPLC. Thus, plasma HPLC data were obtained from a “parent group” of 19 patients receiving 111In-2IT-BAD-Lym-1 and a subgroup of 3 patients providing comparative data for 111In- and 90Y-2IT-BAD-Lym-1.
Patients with metastatic prostate cancer (n = 15) that had become refractory to hormonal therapy entered studies of 111In- and 90Y-2IT-BAD-m170. The mean age was 64 y (range, 54–76 y). The mean Karnofsky performance status was 85 (range, 70–90). m170 reactivity was shown in all patients by positive tumor imaging.
Patients with breast cancer (n = 7) that had progressed despite standard therapy entered studies of 111In- and 90Y-2IT-BAD-m170. The mean age was 45 y (range, 41–50 y). The mean Karnofsky performance status was 81 (range, 70–90). m170 reactivity was shown in all patients by immunohistochemical staining or positive tumor imaging.
Patients were eligible if their serum tested negative for human antimouse antibody (HAMA), they had received no other cancer therapy for at least 4 wk, they had measurable disease at the time of entry, and they met additional trial-specific criteria. Before trial entry, patients signed an informed consent that was approved by the University of California at Davis human subjects and radiation use committees under an investigational new drug authorization from the U.S. Food and Drug Administration.
Antibodies
Lym-1 (Damon Biotechnology, Needham Heights, MA; or Techniclone, Inc., Tustin, CA) is a nonshedding, noninternalizing IgG2a mouse mAb with high affinity for a discontinuous epitope on the β-subunit of human leukocyte antigen (HLA)-DR10, located on the surface membrane of malignant B-lymphocytes (19). m170 (Biomira, Inc., Edmonton, Alberta, Canada) is an IgG1 mouse mAb used in clinical trials for therapy of breast cancer and other adenocarcinomas (20). Biopsy specimens of metastatic breast cancer revealed that 90% of tumors were stained abundantly with m170 (21).
Radiolabeling
2IT-BAD-Lym-1 and 2IT-BAD-m170 were conjugated and radiolabeled with 111In (Mallinckrodt, St. Louis, MO; Amersham, Arlington Heights, IL; or Nordion, Kanata, Ontario, Canada) or 90Y (Pacific Northwest National Laboratory, Richland, WA) as described (13,14). 111In-2IT-BAD-Lym-1, 90Y-2IT-BAD-Lym-1, and 111In-2IT-BAD-m170 were purified and transferred to sterile saline by gel filtration chromatography. Patient doses were filtered (0.22 μm) and formulated at 37 MBq/mL in 4% human serum albumin/saline.
Lym-1 and m170 were radiolabeled with 125I (ICN Radiochemicals, Irvine, CA) as reference standards for radioimmunoassays. 125I-Lym-1 was prepared by the chloramine-T reaction (Sigma Chemical, St. Louis, MO) to a final specific activity of 40 MBq/mg Lym-1. 125I-m170 was prepared with IODO-GEN iodination reagent (Pierce, Rockford, IL) at 350 MBq/mg m170. The specific activities were chosen to provide sufficient counts for the assays.
Quality Assurance
Radiopharmaceuticals were evaluated before injection for structural and functional integrity by HPLC, cellulose acetate electrophoresis (CAE), and radioimmunoassay, except the radioimmunoassay of 111In-2IT-BAD-m170 was performed retrospectively on decayed samples. HPLC of radiopharmaceuticals (Beckman, Fullerton, CA) was performed using a gel filtration column (Biosep-SEC 3000; Phenomenex, Torrance, CA [or equivalent]) eluted in 0.1 mol/L sodium phosphate buffer, pH 7, at a flow rate of 1.0 mL/min. HPLC traces were monitored by ultraviolet absorbance (280 nm) and radioanalytic detection.
CAE (Gelman Sciences, Inc., Ann Arbor, MI) was performed using 0.05 mol/L sodium barbital buffer, pH 8.6. A current of 5 mA per strip was applied. Samples were electrophoresed for 11 min to resolve small species (i.e., free radiochelates) from immunoconjugates and 45 min to resolve high MW species (e.g., immunoconjugate aggregates) from monomers. Detection and quantitation were by radiation scanning (AMBIS, San Diego, CA).
The immunoreactivity of 111In- and 90Y-2IT-BAD-Lym-1 was assessed by solid-phase radioimmunoassay against partially purified membrane fragments from Raji cells as described (22). The immunoreactivity of 111In-2IT-BAD-m170 was assessed by a competitive assay against rabbit anti-m170 idiotype antibody. The rabbit anti-m170 idiotype antibody binds m170 near the m170-antigen binding site; thus, its ability to bind m170 indicates that the m170-antigen binding site is intact. Decayed 111In-2IT-BAD-m170 (0, 1.25, 2.5, 5.0, 10, 20, or 40 ng) and 125I-m170 (4 ng) were incubated in a test tube coated with rabbit anti-m170 idiotype antibody for 2 h at room temperature. The fraction of 125I-m170 bound in the absence of 111In-2IT-BAD-m170 was the maximum binding fraction. The amount of decayed 111In-2IT-BAD-m170 required to reduce 125I-m170 binding to half of the maximum binding fraction (ED50) was determined. Thus, a smaller ED50 indicated higher immunoreactivity. A parallel study was performed, in which the ED50 of the m170 reference standard was assessed. The relative immunoreactivity of 111In-2IT-BAD-m170 was calculated as the quotient, ED50 reference standard/ED50 111In-2IT-BAD-m170.
Antibody Injection
Unmodified Lym-1 or m170 was given before radiolabeled Lym-1 or m170, respectively, to block nonspecific binding sites and provide stable pharmacokinetics (23). The total unmodified plus radiolabeled antibody injected, at a rate of 0.5–1.0 mg/min, was 6.3–31 mg in patients receiving 111In-2IT-BAD-Lym-1 and 5.1–11 mg in patients receiving 111In-2IT-BAD-m170, depending on the specific activity of the radiopharmaceutical.
Analysis of Blood and Urine
Blood samples were obtained immediately, during the next 6 h, and daily up to 7 d after injection of radiopharmaceuticals. Blood was collected in Vacutainer test tubes that were coated with buffered sodium citrate to prevent clotting (Becton Dickinson, Franklin Lakes, NJ). Blood samples (0.1 or 0.5 mL) were counted to obtain the concentration of 111In or 90Y in the blood. The remainder of the blood was centrifuged, the plasma was decanted, and fresh plasma samples (0.1 mL) were examined by HPLC, as described, on the day received. HPLC eluant fractions (0.5 mL) were collected (Gilson, Inc., Middleton, WI), and their activities were measured in a γ-well counter (Pharmacia Biotech, Inc., Piscataway, NJ). HPLC traces of unmodified Lym-1 and m170 indicated an elution time of 9.9 ± 0.2 min (i.e., apparent MW, 147 ± 25 kilodaltons [kDa]) based on a standard curve using MW standard proteins (Bio-Rad, Hercules, CA). The correspondence between elution volume and MW according to the standard curve is shown in Figure 1. To allow for variability in HPLC performance, individual radioanalytic HPLC traces and fraction counts were evaluated to designate peaks as complexes, monomers, and metabolites. Generally, HPLC fractions collected over the range 7–9 mL, 9–12 mL, and 12–18 mL were designated as complexes, monomers, and metabolites, respectively.
Radioanalytic HPLC traces of 111In-2IT-BAD-Lym-1 radiopharmaceutical before injection in NHL patient (front trace) and of patient plasma 24, 48, and 72 h after injection. Percentage of 111In in patient’s plasma associated with metabolites (elution volume, 12–18 mL) and complexes (elution volume, 7–9 mL) increased over time. Similar pattern was observed for every NHL patient who received 111In-2IT-BAD-Lym-1. Standard curve from MW standard proteins was used to relate elution volume to MW.
Immunoprecipitation studies were performed to further characterize the radioactive species in plasma, specifically, to test for the transchelation of radiometals to serum proteins. Affinity-purified antialbumin and antitransferrin antibodies (Sigma) were added to plasma from an NHL patient receiving 111In-2IT-BAD-Lym-1, and from an NHL patient receiving 90Y-2IT-BAD-Lym-1, and precipitated as described (16).
Urine was collected up to 7 d after infusion of radiopharmaceuticals. Aliquots of urine (0.1 mL) were assayed in a γ-well counter and then multiplied by the measured urine volume to calculate output of 111In or 90Y.
Pharmacokinetics
The cumulated activity of 111In in the blood was obtained by fitting the percentage injected dose (%ID) of 111In in the blood versus time to a biexponential function (13). The cumulated activity in the blood from monomeric 111In-2IT-BAD-Lym-1 was also calculated as follows. At each time point, the %ID of 111In in the blood was multiplied by the fraction of 111In as monomeric 111In-2IT-BAD-Lym-1 from HPLC analysis of plasma. These data, %ID of 111In as monomeric 111In-2IT-BAD-Lym-1 versus time, were fitted to a biexponential function. The cumulated activities of 90Y in the blood, from all species and from monomeric 90Y-2IT-BAD-Lym-1, were calculated by similar methods.
Methods for obtaining pharmacokinetic data for 111In-2IT-BAD-Lym-1 and 111In-2IT-BAD-m170 in organs and tumors have been described (24). Briefly, planar images of conjugate views were acquired immediately, during the next 6 h, and daily for 3–7 d after injection of radiopharmaceuticals and used to quantitate 111In in organs and tumors.
The cumulated activity in tissues was determined by integrating the time–activity curve over time. Pharmacokinetic data in tissues were usually fit to a monoexponential function to calculate cumulated activity. However, in some instances, the uptake in tumor or liver increased continuously over the imaging period of a week; in these instances, the cumulated activities were determined by fitting the data to a cubic spline function up to the last imaging data point and then assuming a constant concentration afterward. Except for the spleen, MIRD reference man masses were used for all organs to obtain cumulated activity concentration (25). Patient-specific splenic cumulated activity concentration was determined using the actual spleen volume measured from CT images (26). The masses of palpable and nonpalpable tumors were determined using calipers and CT or MRI images, respectively. Tumors <2 g by caliper measurement, or <10 g by CT measurement, were excluded from the analysis to ensure accuracy. Lumbar vertebrae marrow imaging was used to calculate the cumulated activity in the marrow (27). The uptake of 111In in 3 lumbar vertebrae was extrapolated to uptake in total marrow, assuming that the red marrow mass in the 3 lumbar vertebrae constituted 6.7% of total red marrow mass (28).
TIs
To calculate 90Y radiation dosimetry, pharmacokinetic data for 90Y-2IT-BAD-Lym-1 were assumed to be the same as those for 111In-2IT-BAD-Lym-1 (29). The AUC for each tissue was converted to radiation dose using MIRD formulas as reported in detail (13). TIs were calculated by dividing the radiation dose to the tumor by the radiation dose to each of the normal tissues. In the case of radionuclide therapy, TIs reflect relative tumor and normal tissue effect, albeit lacking radiobiologic factors, such as tissue radiosensitivity.
Chelate Stability and Formation of Metabolites In Vitro
Previously, 88Y-2IT-BAD-Lym-1 showed no measurable decomposition in human serum under physiologic conditions for 25 d (1). For this study, the stability of 114mIn-2IT-BAD-Lym-1 in human serum was evaluated by similar methods; 88Y and 114mIn were used as long-lived analogs of 90Y and 111In to allow prolonged studies. Briefly, 114mIn-2IT-BAD-Lym-1 was prepared using methods for 111In-2IT-BAD-Lym-1 radiolabeling as above. 114mIn-2IT-BAD-Lym-1 was added to human serum from a volunteer such that the concentrations of 114mIn, DOTA, and Lym-1 were 1.9 MBq/mL, 2 μmol/L, and 0.5 μmol/L, respectively. Duplicate solutions were incubated at 37°C in a humidified, 5% carbon dioxide/95% air atmosphere, and a pH of 7.4 ± 0.1 was maintained. Aliquots (10 μL) were removed at 0, 0.83, 1.8, 4.7, 7.1, and 13.8 d and evaluated by native polyacrylamide gel electrophoresis and radioanalytic imaging, as described (1), to determine the fraction of 114mIn transchelated to serum proteins. Control solutions of 114mIn-2IT-BAD-Lym-1 in water and free 114mIn in serum were also electrophoresed at every time point to calibrate the migration of all 114mIn-labeled species.
To investigate possible mechanisms for the metabolism of 111In-2IT-BAD-Lym-1, as observed in clinical studies, 111In-2IT-BAD-Lym-1 was added to plasma from 2 NHL patients and a volunteer and monitored for formation of metabolites and complexes in vitro. Blood was collected in Vacutainer test tubes coated with buffered sodium citrate. The blood was centrifuged, and plasma was decanted and filtered (0.45 μm). 111In-2IT-BAD-Lym-1 was added to each plasma sample such that the final concentrations of 111In, DOTA, and Lym-1 were 0.37 MBq/mL, 0.023 μmol/L, and 0.017 μmol/L, respectively. The solutions were incubated at 37°C in a humidified, 5% carbon dioxide/95% air atmosphere. Aliquots (100 μL) were removed at 0, 1, 2, 3, and 7 d and evaluated by molecular sieving HPLC to determine the fraction of 111In-2IT-BAD-Lym-1 in the forms of monomer, metabolites, and complexes.
To investigate the role of lymphoma cells in the metabolism of 111In-2IT-BAD-Lym-1, the radiopharmaceutical was added to Raji cell culture and monitored for the formation of metabolites and complexes in vitro. Raji is an American Type Culture Collection–certified cell line, established in 1963 from a Burkitt’s lymphoma of the left maxilla of an 11-y-old boy. Three assay cultures were prepared as follows. 111In-2IT-BAD-Lym-1 (15 kBq/110 ng) was added to Raji cells (3 × 106) in medium (0.5 mL) of 10% fetal calf serum/RPMI medium 1640 (GIBCO BRL, Grand Island, NY). The cultures were incubated for 2 h at 37°C in a humidified, 5% carbon dioxide/95% air atmosphere, washed 3 times in medium, and resuspended in medium (0.5 mL). The wash eluants and assay cultures were counted to determine the fraction of 111In-2IT-BAD-Lym-1 bound to the Raji cells. The assay cultures were incubated at 37°C in a humidified, 5% carbon dioxide/95% air atmosphere. At 4 h, 1 assay culture was centrifuged and washed, and the cell pellet and supernatant were counted to determine the fraction of 111In-2IT-BAD-Lym-1 bound to Raji cells. The supernatant was evaluated by HPLC to determine the fraction of 111In-2IT-BAD-Lym-1 in the forms of monomer, metabolites, and complexes. The remaining assay cultures were evaluated similarly at 1 and 2 d. A similar study was performed with a smaller amount of 111In-2IT-BAD-Lym-1 (3.7 kBq/27 ng). Similar studies were performed with 125I-Lym-1 (15 kBq/400 ng and 3.7 kBq/100 ng). Control studies were performed as described, except Raji cells were excluded. Cultures of Raji cells (3 × 106) in medium (0.5 mL) were assessed for viability by exclusion of trypan blue (Sigma) at all time points.
RESULTS
Radiopharmaceuticals
Patient doses of 111In-2IT-BAD-Lym-1, 90Y-2IT-BAD-Lym-1, and 111In-2IT-BAD-m170 showed high structural and functional integrity before injection (Table 1). Similar results were obtained for RICs prepared for in vitro experiments.
Radiopharmaceutical Quality Assurance Results
Metabolites and Complexes
HPLC of 111In-2IT-BAD-Lym-1 radiopharmaceuticals before injection indicated a single peak corresponding to monomeric mAb, but after injection HPLC of patient plasma indicated substantial fractions of metabolites and complexes, which increased over time (Fig. 1). Metabolites and complexes of 111In-2IT-BAD-Lym-1 were observed in the plasma of 18 of 19 NHL patients within 1 d of injection and 19 of 19 patients within 3 d. Three days after injection of 111In-2IT-BAD-Lym-1, a mean ± 1 SD of 1.3 ± 1.4 %ID of 111In was in the patients’ blood, after correction for decay, of which monomer, metabolites, and complexes constituted 54% ± 20%, 36% ± 18%, and 10% ± 5.9%, respectively. The extent of metabolism of 111In-2IT-BAD-Lym-1 was not related to splenomegaly, circulating lymphoma cells, histologic marrow lymphoma, or amount of Lym-1 injected.
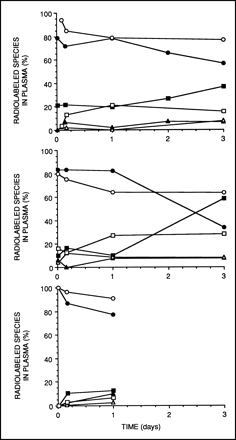
The apparent metabolism of 111In-2IT-BAD-Lym-1 in NHL patients occurred earlier and to a much greater extent than that of 111In-2IT-BAD-m170 in breast and prostate cancer patients (Fig. 2). Three days after injection of 111In-2IT-BAD-m170 in patients with breast cancer, a mean of 29.2 ± 5.4 %ID of 111In was in the patients’ blood, after correction for decay, of which monomer constituted 91% ± 8.8%. The corresponding values for prostate cancer patients were 26.4 ± 3.6 %ID at 3 d, of which 94% ± 12% was monomer. In 12 of 22 breast and prostate cancer patients, no metabolites or complexes of 111In-2IT-BAD-m170 were observed 3 d after injection.
Comparison of metabolism of 111In-2IT-BAD-Lym-1 in NHL patients (A), 111In-2IT-BAD-m170 in prostate cancer patients (B), and 111In-2IT-BAD-m170 in breast cancer patients (C). Of 111In-2IT-BAD-Lym-1 or 111In-2IT-BAD-m170 in patients’ plasma at specific points in time, percentage in monomeric (•), metabolite (▪), and complexed (▴) forms was measured by HPLC. In NHL patients receiving 111In-2IT-BAD-Lym-1, metabolites and complexes constituted substantially larger fractions of 111In in plasma compared with prostate and breast cancer patients receiving 111In-2IT-BAD-m170. Decay-corrected mean percentage injected dose (%ID) of 111In in patients’ blood decreased over time (dashed lines). Thus, metabolites and complexes of 111In-2IT-BAD-Lym-1 showed relative growth over time as fractions of total 111In activity, but not absolute growth in terms of %ID.
A comparison of 111In-2IT-BAD-Lym-1 and 90Y-2IT-BAD-Lym-1 in the 3 evaluable patients who received both showed that, in individual patients, the formation of 111In and 90Y metabolites and complexes occurred at similar rates (Fig. 3). The immunoprecipitation studies indicated that transchelation of 111In or 90Y from 2IT-BAD-Lym-1 to albumin or transferrin did not occur.
Comparison of formation of metabolites and complexes of 111In-2IT-BAD-Lym-1 and 90Y-2IT-BAD-Lym-1. Each graph represents 1 NHL patient who received 111In- and 90Y-2IT-BAD-Lym-1 in close temporal proximity. Of 111In-2IT-BAD-Lym-1 in patients’ plasma at specific points in time, percentage in monomeric (•), metabolite (▪), and complexed (▴) forms was measured by HPLC. Of 90Y-2IT-BAD-Lym-1 in patients’ plasma at specific points in time, percentage in monomeric (○), metabolite (□), and complexed (▵) forms was measured by HPLC. Extent of formation of nonmonomeric forms of 111In- and 90Y-2IT-BAD-Lym-1 was similar in individual patients.
Pharmacokinetics and TIs
In patients receiving 111In-2IT-BAD-Lym-1, the mean values of 111In in the blood were 6.0 ± 7.0, 1.8 ± 2.0, 1.3 ± 1.4, and 0.26 ± 0.14 %ID at 1, 2, 3, and 7 d after injection, respectively. 111In cleared the blood with a β-phase intercept and β-phase half-time of 16% ± 20% and 1.5 ± 1.7 d, respectively. The mean blood AUC was 0.83 kBq . h/g/MBq injected. The contribution of 111In-2IT-BAD-Lym-1 monomer to the total blood AUC was calculated. 111In-2IT-BAD-Lym-1 monomer cleared the blood with a β-phase intercept and β-phase half-time of 16% ± 20% and 1.0 ± 0.7 d, respectively. The mean blood AUC from 111In-2IT-BAD-Lym-1 monomer was 0.75 kBq.h/g/MBq. Thus, because monomeric 111In-2IT-BAD-Lym-1 was the predominant species in blood at early time points and contributed 90% of the AUC (0.75/0.83), the pharmacokinetic parameters of all 111In in the blood and monomeric 111In-2IT-BAD-Lym-1 in the blood were similar. However, 111In in the blood associated with nonmonomeric species grew over time and provided 10% of total blood AUC, a substantial fraction.
For every patient who received 111In/90Y-2IT-BAD-Lym-1, the AUC for tumor was greater than the AUC for marrow, kidneys, and lungs. However, in many patients, the AUC for liver or spleen was greater than that for tumor (Table 2). TIs for 90Y-2IT-BAD-Lym-1 were tumor/total body, 13.6; tumor/marrow, 3.7 (lumbar imaging method); tumor/liver, 1.2; tumor/lungs, 5.3; tumor/kidneys, 2.5; and tumor/blood, 15.7.
AUC of 111In (kBq.h/g of Tissue per MBq Injected) from 111In-2IT-BAD-Lym-1
The mean cumulative excretion of 111In in urine was 4.5 ± 2.2, 12.7 ± 4.7, and 21.2 ± 6.8 %ID of 111In-2IT-BAD-Lym-1 at 1, 2, and 3 d, respectively.
In Vitro Studies
Polyacrylamide gel electrophoresis of 114mIn-2IT-BAD-Lym-1 in serum from a volunteer showed that 98.0%, 98.1%, 98.4%, 97.5%, 97.3%, 96.9%, and 96.5% of 114mIn was associated with 2IT-BAD-Lym-1 at 0, 0.83, 1.8, 4.7, 7.1, 10.2, and 13.8 d, respectively, indicating a loss of 114mIn at a rate of 0.13% per day by transfer to serum proteins or cleavage from Lym-1 antibody.
111In-2IT-BAD-Lym-1 added to the plasma of 2 NHL patients in vitro formed metabolites and complexes similar to those of the volunteer and much less than in vivo (Table 3). At 3 d, 92.6%, 89.1%, and 85.8% of 111In was associated with monomer in the plasma of the 2 NHL patients and the volunteer, respectively, in the in vitro study. Comparatively, at 3 d, a mean of only 54.0% of 111In was associated with monomer in the plasma of all patients in vivo.
111In-2IT-BAD-Lym-1 in Plasma of 1 Volunteer and 2 NHL Patients In Vitro: % Monomer/ % Metabolites/% Complexes
111In-2IT-BAD-Lym-1 in Raji cell culture was examined. The Raji cells were at least 94% viable up to 48 h. The following data pertain to the Raji cell cultures containing 15 kBq 111In-2IT-BAD-Lym-1, but similar results were obtained in the 3.7-kBq studies. Most 111In remained associated with the Raji cells, 79% at 48 h (Table 4). The remainder of the 111In, dissociated from the Raji cells and in the supernatant, was in the forms of monomer, 14.8%; metabolite, 5.1%; and complex, 1.1%. 125I-Lym-1 showed greater formation of metabolites, and the 125I-Lym-1 metabolites were smaller in size (data not shown).
Processing of 111In-2IT-BAD-Lym-1 and 125I-Lym-1 in Raji Cell Culture
DISCUSSION
Metabolites of iodinated antibodies and, less frequently, radiometal-labeled antibodies have been reported in patient urine but, to our knowledge, not in patient plasma; therefore, the observation of radiolabeled metabolites of 111In/90Y-2IT-BAD-Lym-1 in patient plasma was unexpected. It is not apparent whether the absence of reports of metabolites in the plasma of patients receiving 111In- or 90Y-labeled antibodies reflects the fact that metabolites are uncommonly formed or that diagnostic assays are uncommonly performed or reported. In earlier studies, metabolites of 131I-Lym-1 or 67Cu-2IT-BAT-Lym-1 were generally not observed in patient plasma using HPLC, although it is possible that they were formed and then rapidly excreted.
The fraction of radiolabeled metabolites of 111In-2IT-BAD-Lym-1 in plasma grew to 36% at 3 d, and 90Y data were similar. The fraction of total radiopharmaceutical that had undergone metabolism up to that time was likely different because plasma represents a single compartment in a dynamic system: Nearly 99% of the injected dose of 111In had cleared the blood in 3 d, species entered and left the plasma and related interstitial space, and the pharmacokinetics of metabolites and whole antibody were likely different. Regardless, on the basis of frequent sampling and analysis of patient plasma, nonmonomeric species contributed a mean 10% of cumulated activity in the blood (kBq.h/mL plasma per MBq injected). The nonmonomeric fraction was less likely to be immunoreactive or capable of active tumor uptake, resulting in lower TIs for 111In/90Y-2IT-BAD-Lym-1. TIs for the total body, marrow, liver, kidneys, spleen, and blood for 111In/90Y-2IT-BAD-Lym-1 were lower than those reported for 67Cu-2IT-BAT-Lym-1 (30), suggesting that metabolism of 111In/90Y-2IT-BAD-Lym-1 had an adverse effect on the TI.
The metabolism of 111In/90Y-2IT-BAD-Lym-1 may affect its uptake and retention in tumors and normal tissues. Complexes may or may not retain immunoreactivity but are likely to be extracted by the phagocytic cells of the reticuloendothelial system of the liver, spleen, bone marrow, and elsewhere. Metabolites are often readily excreted through the kidneys or, less commonly, the gastrointestinal (GI) tract but may be trapped (dependent on the nature and size of the metabolites) by the renal tubular cells or other tissues, particularly when the metabolites are radiometal labeled (Fig. 4). Generally, 111In was readily visualized in the GI tract of the NHL patients who received 111In-2IT-BAD-Lym-1 but not the breast and prostate cancer patients who received 111In-2IT-BAD-m170 (Fig. 5).
Simplified model for distribution of 111In/90Y-2IT-BAD-Lym-1 RIC in circulation in blood. Monomeric RIC is preferentially distributed to tumor, whereas metabolites and complexes of RIC are excreted or distributed to normal tissues. Thus, formation of metabolites and complexes of 111In/90Y-2IT-BAD-Lym-1 resulted in reduced cumulated activity in tumor and increased cumulated activity in normal tissues (lower TIs and higher toxicity).
Planar anterior view of pelvis of NHL patient given 111In-2IT-BAD-Lym-1 (A) and breast (B) and prostate (C) cancer patients given 111In-2IT-BAD-m170, 3 d after injection. In addition to 111In in lymphoma (←) and marrow (←), 111In was present in GI tract () of patient with NHL but not in patients with breast and prostate cancer. Specific hepatocyte receptors have been documented for mouse IgG2a antibodies, such as Lym-1, but not for mouse IgG1 antibodies, such as m170.
The data support processing of 111In/90Y-2IT-BAD-Lym-1 in the hepatocytes as the dominant mechanism for the production of metabolites. Antibody processing with metabolite induction usually occurs in the hepatocytes; specific hepatocyte receptors have been documented for mouse IgG2a antibodies, such as Lym-1 (31,32), but not for mouse IgG1 antibodies, such as m170, consistent with the presence of 111In-2IT-BAD-Lym-1 metabolites and the absence of 111In-2IT-BAD-m170 metabolites in patient plasma. Hepatocyte processing is supported by the imaging evidence for 111In in the GI tract of patients given Lym-1 (Fig. 5). The observation of a significant metabolite fraction in plasma soon after injection (Fig. 2A) indicates the involvement of a well-perfused system in the formation of metabolites. All NHL patients received an amount of unlabeled Lym-1 that was documented in earlier studies to be sufficient to saturate the Lym-1 receptor on hepatocytes (31). It is conceivable that the hepatocytes were able to discriminate the 111In- or 90Y-2IT-BAD-Lym-1 from the unlabeled Lym-1, thereby permitting uptake and metabolism. However, processing by hepatocytes does not explain the 111In/90Y-2IT-BAD-Lym-1 complexes in the plasma, which accounted for about one fourth of the nonmonomeric species. Because of the practical and conceptual importance of definition of the mechanism for formation of the metabolites, hepatocyte involvement should be investigated further—specifically, the effect of chemical modification of antibody for interaction with hepatocyte molecules.
Other possible mechanisms of formation of the nonmonomeric radiolabeled 111In and 90Y species in the plasma were considered, evaluated against clinical data, and, in some cases, investigated further in vitro, specifically: instability of the 111In and 90Y RICs; complexation by antigen or HAMA in the plasma and extraction with metabolism by phagocytic cells; and uptake, internalization, and metabolism by lymphoma cells or normal B-lymphocytes.
The exceptional stability of the chelate moiety and the conjugate linker of In- and Y-2IT-BAD-Lym-1 in human serum under physiologic conditions in vitro has been shown here and elsewhere (1). The stability of 111In- and 90Y-2IT-BAD-m170 in the plasma of patients with breast or prostrate cancer in vivo also corroborated the stability of the chelate and conjugate linker. Finally, immunoprecipitation studies of plasma of patients who had received 111In- and 90Y-2IT-BAD-Lym-1 indicated that transchelation of 111In or 90Y from 2IT-BAD-Lym-1 to albumin or transferrin did not occur in vivo. In evaluating specifically for the metabolism of 111In-2IT-BAD-Lym-1 in vitro, metabolites and complexes were formed to a minor extent in lymphoma plasma in vitro, similar to volunteer plasma (Table 3), and the in vitro results were quantitatively insufficient to explain the in vivo events observed in the NHL patients. Because all NHL patients tested negative for HAMA, complexation and phagocytosis with subsequent metabolism were also unlikely.
Lastly, the possible uptake and metabolism of 111In/90Y-2IT-BAD-Lym-1 by normal or malignant NHL lymphocytes (or both) were considered. Two patients who exhibited substantial metabolite fractions in plasma, despite lacking a pool of normal B-lymphocytes, provide anecdotal evidence against normal lymphocytes. One patient had no spleen and the second patient had B-lymphocyte depletion associated with earlier rituximab (Rituxan; IDEC Pharmaceuticals Corp., San Diego, CA) therapy. Furthermore, the HLA-DR antigen is low in density on the surface membrane of normal B-lymphocytes and has not been observed to be a source of significant Lym-1 binding (33). In the case of malignant NHL lymphocytes, the degree of internalization of Lym-1 in Raji human Burkitt’s lymphoma cells or NHL cells has been variously reported as low (34) to quite high, followed by rapid metabolism and excretion (35). However, in the latter report, 111In-radiolabeled Lym-1 was residualized in Raji cells. Our study indicated that, at 48 h, only 5% of 111In-DOTA-2IT-BAD-Lym-1 was released as metabolites by Raji cells in vitro and did not support substantial lymphocyte involvement in the metabolite formation observed in patients. Most but not all NHL patients had large tumor burdens, but no relationship between tumor burden and metabolite formation was found. A few patients had quite small tumor burdens yet had substantial metabolite formation.
The observation of metabolites of 111In/90Y-2IT-BAD-Lym-1 in patient plasma, but not of 131I-Lym-1 or 67Cu-2IT-BAD-Lym-1, indicates the remarkable influence of the radiolabel on the cellular processing of radiolabeled antibodies. It is well documented that degraded radiometal-labeled antibodies residualize in cells, but iodinated antibodies are degraded to small species (i.e., iodotyrosine) that are rapidly excreted. It has been shown that 67Cu-ceruloplasmin is a major degradation or transcomplexation product of 67Cu-2IT-BAT-Lym-1. With a MW of 150 kDa, 67Cu-ceruloplasmin in patient plasma was indistinguishable from 67Cu-2IT-BAD-Lym-1 by HPLC but was identified by immunoprecipitation studies (16).
Cellular processing of iodinated and radiometal-labeled mAbs has been investigated in cell culture and animal models (4–6,32,35–39); similar studies of 111In/90Y-2IT-BAD-Lym-1, though beyond the scope of this investigation, may be instructive. Although these observations have potential importance for all RICs and emphasize the need to document the nature of the radiolabeled species in the plasma (and urine and stool), they may not necessarily be extrapolated to other RICs. The discrepant results for the other radiolabeled mAb, 111In- and 90Y-2IT-BAD-m170, are probably more representative of the common circumstance. Juweid et al. (40) have shown substantial improvement in TIs for 111In- or 90Y-labeled humanized LL2 anti-CD22 mAb compared with 131I-labeled mAb in NHL patients, although results for HPLC or immunoreactivity were not reported.
CONCLUSION
Molecular sieving HPLC indicated the presence of metabolites and complexes in the plasma of every NHL patient soon after injection of 111In- and 90Y-2IT-BAD-Lym-1, despite the stability of these RICs in vitro. Metabolism of 90Y-2IT-BAD-Lym-1 in the NHL patients appeared to reduce TIs for radiation dose. Clinical data and subsequent in vitro studies suggested processing of 111In/90Y-2IT-BAD-Lym-1 in the hepatocytes as the dominant mechanism for the production of metabolites.
Acknowledgments
This research was supported by National Cancer Institute grants PO1 CA47829 and CA16861, Berlex Research Agreement 94K058, and Department of Energy grant DE FG03-84ER60233.
Footnotes
Received Nov. 17, 2000; revision accepted May 2, 2001.
For correspondence or reprints contact: Gerald L. DeNardo, MD, Molecular Cancer Institute, 1508 Alhambra Blvd., Suite 3100, Sacramento, CA 95816.