Abstract
Previously, sympathetic reinnervation of the transplanted heart has been described using invasive catheterization techniques and noninvasive radionuclide imaging techniques. However, little is known about the agreement between these 2 methods. Thus, correlation between 11C-hydroxyephedrine (HED) PET and invasively measured norepinephrine (NE) release was investigated in transplant recipients in this study. Methods: Using PET and the catecholamine analog HED, 17 patients were studied between 2 mo and 13.6 y after transplantation. Based on results in completely denervated hearts, areas with HED retention >7%/min were defined as reinnervated. Additionally, transcardiac NE release induced by intravenous tyramine (55 μg/kg) was measured by coronary sinus and aortic catheterization within 1 wk of the PET study. NE levels between coronary sinus and aortic root, ΔNECS–AO, were calculated at baseline and after tyramine administration. Differences of more than 3 SD of baseline (>163 pg/mL) were interpreted as reinnervation. Results: HED retention indicated reinnervation in 10 patients. Maximal HED retention ranged from 4.3%/min to 16.4%/min. ΔNECS–AO 1 min after tyramine administration ranged between −10 pg/mL and 1157 pg/mL, and 8 patients were above the reinnervation threshold. Fisher’s exact test demonstrated good agreement between results of PET and ΔNECS–AO measurements (P = 0.002). Maximal HED retention was also significantly correlated with NE release (r = 0.69; P = 0.001). Conclusion: Results of invasively measured NE release and noninvasive 11C-HED PET are well correlated. This study further supports the usefulness of PET as a noninvasive approach for detection of reappearance of catecholamine uptake sites after heart transplantation.
The surgical procedure of heart transplantation causes autonomic denervation of the donor heart, as demonstrated by axonal degeneration and depletion of norepinephrine (NE) stores (1). Re-establishment of functional reinnervation in animals after transplantation and its clinical relevance have been reported (2,3). Furthermore, tyramine-induced transcardiac NE release was shown invasively in patients after heart transplantation and interpreted as a sign of sympathetic reinnervation (4–8). Recently, noninvasive scintigraphic studies using 123I-metaiodobenzylguanidine (MIBG) or 11C-hydroxyephedrine (HED) have shown the regional recurrence of NE uptake sites in the human heart (9–14). These results, independently derived from invasive and noninvasive methods, indicate that regional reappearance of sympathetic nerve fibers occurs in the human transplanted heart with functional integrity of the nerve terminals. However, little is known about the correlation between these techniques (15).
The purpose of this study was to compare results of 11C-HED PET with invasively measured NE release after transplantation. PET-measured intensity and extent of NE uptake sites were correlated intraindividually with invasive tyramine-induced transcardiac release of NE to document the pharmacologic integrity of sympathetic nerve terminals.
MATERIALS AND METHODS
Patients
Patients were selected out of a population of patients (n = 421) in whom transplantation had been performed since August 1981. Selection was performed to cover varying time intervals after transplantation (range, 2 mo to >10 y) and to meet inclusion and exclusion criteria. The patients were approached during their yearly follow-up examinations and underwent PET based on scanner availability and each patient’s residency. Patients had to be older than 18 y. Those receiving medication that interfered with catecholamine uptake (e.g., tricyclic antidepressant) or presenting with diabetes mellitus or cardiac allograft vasculopathy were excluded. None of the patients showed any signs of acute rejection as documented by endomyocardial biopsy 24–48 h before the PET study.
Seventeen individuals (16 men, 1 woman; age range, 37–62 y; mean age, 50.6 ± 8.4 y) were investigated by PET between 2 mo and 13.6 y after transplantation. Dilated cardiomyopathy had been diagnosed in 11 patients, whereas 6 patients suffered from ischemic heart disease before transplantation. Mean left ventricular ejection fraction was 75% ± 13%, as determined in 14 patients by left ventriculography. Although 4 patients had no previous rejection episodes, 8 patients had 1 or 2 and 5 patients had >2 documented rejection episodes. All patients received immunosuppressive therapy consisting of cyclosporine A, azathioprine, and prednisone. Induction therapy using antithymocyte globulin had been performed perioperatively in 13 patients.
All patients signed informed consent forms before participating in the study. The Technische Universität München ethical committee approved the protocol.
PET Imaging
The radiolabeled catecholamine analog 11C-HED was synthesized according to Rosenspire et al. (16). Imaging was performed with a 951R PET scanner (CTI/Siemens, Knoxville, TN). First, 13N-ammonia (370 MBq) was used for qualitative assessment of relative myocardial perfusion (17). After waiting for physical decay of 13N, 11C-HED (565–740 MBq) was injected as a slow bolus over 30 s followed by a dynamic acquisition of 14 frames over 40 min (9).
Attenuation-corrected transaxial images were reconstructed by filtered backprojection using a Hanning filter with 0.3 cycle/bin cutoff frequency, resulting in a spatial resolution of 8–9 mm full width at half maximum. A combination of cylindric and hemispheric volumetric sampling was used to quantify regional tracer distribution (18). From the dynamic PET images, retention was defined as HED activity at 40 min divided by the integral of the blood activity curve (19). Based on results in completely denervated hearts, areas with HED retention below 7%/min were defined as denervated. The threshold of innervation in the human heart was ≥7%/min (20). Maximal left ventricular HED retention was chosen to define the intensity of reinnervation. The fraction of left ventricular myocardium that showed HED retention above this threshold was used as a parameter to define the extent of reinnervation.
Assessment of NE Release
Pharmacologically induced release of NE from large storage vesicles by tyramine has been shown to identify neuronal tissue (21). In this study, tyramine-induced NE release into cardiac veins was measured by coronary sinus and aortic root catheterization in all patients within 1 wk of the PET study.
Simultaneous coronary sinus and aortic root blood samples were taken at baseline and at 1, 3, and 5 min after 55 μg/kg intravenous tyramine injection (4). Plasma NE levels were measured using the high-performance liquid chromatography method. The transcardiac release of NE levels between coronary sinus and aortic root, ΔNECS–AO, was calculated at baseline and after tyramine. Transcardiac NE release after tyramine administration was compared with the average of baseline measurements; a difference of more than 3 times the baseline SD (>163 pg/mL) was interpreted as a sign of innervation.
Statistical Analysis
Values were expressed as mean ± SD. The mean differences for continuous variables were compared using an unpaired Student t test, and the differences for categoric variables were compared using a χ2 test. Correlation between continuous variables was described by Pearson’s correlation coefficient and tested for significance by Fisher’s r to z transformation. Fisher’s exact test was used to determine the concordance of innervation between HED retention and NE release. P < 0.05 was defined as significant.
RESULTS
PET
Relative regional 13N-ammonia values were within 2.5 SDs of normal perfusion in all patients, excluding the presence of regional perfusion defects. By contrast, HED uptake was absent or heterogeneous.
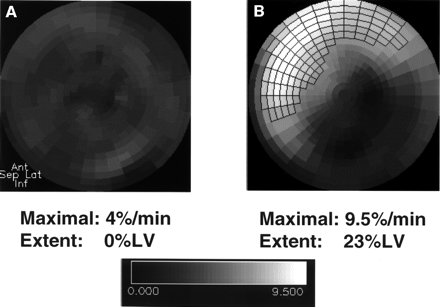
Maximal HED retention was 8.9% ± 4.0%/min (range, 4.3%–16.4%/min) and regional extent of the reinnervated area (>7%/min) was 12.3% ± 17.3%LV (range, 0–66%LV). In 10 patients (59%), areas of regional HED retention >7%/min were identified, indicating sympathetic reinnervation. In all cases with positive HED findings, tracer uptake was located in the anteroseptal wall (Fig. 1).
Dynamic analysis of polar maps of myocardial HED retention. Examples of patients after heart transplantation: denervated heart (A) and reinnervated heart (B). Area of reinnervation is marked with frames. Denervated area comprises inferior wall and parts of lateral wall.
NE Release
Intravenous tyramine injection caused prompt rises in systemic hemodynamics (Table 1). However, tyramine injection also induced cardiac NE release. It increased from baseline 4 ± 53 pg/mL (range, −56 to 186 pg/mL) to 250 ± 316 pg/mL (range, −10 to 1157 pg/mL) (Fig. 2) at 1 min after tyramine injection. In previous studies, maximum NE release was consistently demonstrated at 1 min after tyramine injection (4). Individual values for aortic root and coronary sinus under tyramine stimulation are shown in Figure 3, supporting specific cardiac NE release. Eight patients had tyramine-induced NE release above the reinnervation threshold (163 pg/mL).
Increase of NE release at baseline and 1 min after tyramine injection. Horizontal dashed line represents limit of reinnervation.
NE concentration at ascending aorta and coronary sinus of patients 1 min after tyramine injection.
Effects of Intravenous Tyramine on Hemodynamics
Correlation of Scintigraphic and Functional NE Parameters
Using the HED signal, the patients were grouped based on heart denervation or reinnervation, and the functional NE parameters in these 2 groups were compared. NE release after tyramine injection was significantly higher in the reinnervated group (Table 2). Probably because of low sample numbers, a significant correlation between time after transplantation and HED retention or NE release was not seen.
Average Values in HED Negative and Positive Groups
Figure 4 shows regression plots with significant correlations between NE release after tyramine injection and PET-derived signs of reinnervation concerning both intensity (r = 0.69; P = 0.001 [Fig. 4A]) and extent (r = 0.86; P < 0.001 [Fig. 4B]). The plots identify 2 outlying data points with high NE release. Even when these 2 patients were excluded from the analysis, correlation between NE release and HED reinnervation concerning both intensity (r = 0.82; P < 0.001 [Fig. 4A]) and extent (r = 0.72; P < 0.001 [Fig. 4B]) remained significant.
(A) Correlation between maximum HED retention (intensity) and tyramine-induced transcardiac NE release. Horizontal dashed line represents limit of reinnervation as maximum HED >7%/min and vertical dashed line represents limit of reinnervation of NE release >163 pg/mL. (B) Correlation between extent area of HED >7%/min and tyramine-induced transcardiac norepinephrine release. Vertical dashed line represents limit of reinnervation of NE release >163 pg/mL.
A good agreement between innervated patients and denervated patients was observed using NE release >163 pg/mL and maximum of HED retention >7%/min as a threshold of reinnervation (Table 3). Although all patients with signs of reinnervation according to tyramine-induced NE release showed evidence of reinnervation in 11C-HED PET, there were 2 patients with reinnervation in 11C-HED PET that did not show signs of reinnervation according to NE release. The 2 patients with disagreement between PET and NE release parameters had values for NE release slightly below reinnervation threshold (118 and 110 pg/mL after tyramine injection). Both patients had dilated cardiomyopathy before transplantation, and previous rejection episodes.
Number of Patients with Functional Parameters Below and Above Threshold
DISCUSSION
In summary, the intensity and extent of NE uptake sites as measured by noninvasive 11C-HED PET were well correlated with invasive tyramine-induced transcardiac release of NE, which was evaluated to document the pharmacologic integrity of sympathetic nerve terminals in patients after heart transplantation. A good agreement of thresholds of reinnervation was shown between HED retention and NE release.
Cardiac transplantation leads to denervation of the donor heart because surgical interruption of the postganglionic sympathetic nerve axon causes rapid depletion of the cardiac neurotransmitter NE within nerve terminals (22). Sympathetic reinnervation, as defined by the return of functioning nerve terminals, can occur only if sympathetic ganglia outside the heart are connected with nerve terminals in the transplanted heart. Animal studies of transplant reinnervation have described the pattern and time course. Reappearance of adrenergic nerves was found in biopsies performed on dogs 1 y after transplantation (1). Dog studies using direct electric stimulation have shown sympathetic reinnervation as early as 74 d after transplantation (23). Functional effects were noted 9–12 mo after transplantation, showing normal response to autonomic nerve stimulation but less than normal catecholamine content in tissue (2). Intravenous tyramine increased the heart rate by more than 5 bpm in dogs with autotransplantation (3).
Tyramine taken up by sympathetic terminals triggers the release of stored NE, which reaches pressor concentrations in the synaptic cleft (24). Because Forman et al. (21) reported that tyramine has been useful in the study of its capacity to release NE from nerve endings in human patients, simultaneous blood sampling from the coronary sinus and aortic root combining intravenous tyramine infusion was applied for several reinnervation studies. Invasively measured NE release was first investigated by Wilson et al. (4) in heart transplant recipients and in healthy volunteers using tyramine. NE release in 8 of the volunteers after tyramine administration was 1943 ± 210 pg/mL, which was higher than in heart transplant patients, even among those who had exhibited signs of reinnervation. Seventy-eight percent of recipients beyond the first year after heart transplantation yielded evidence of significant, though partial, reinnervation, with average NE release of 25% of control values. This pharmacologic method of detecting NE within nerve terminals showed evidence of reinnervation after orthotopic transplantation in humans. However, the question of congruent atrial and ventricular reinnervation was addressed in 45 heart transplant recipients with tyramine-induced increase of heart rate and transcardiac NE release (5). More than 1 y after transplantation, incongruent atrial and ventricular reinnervation was found in about half of the innervated patients.
Evidence of sympathetic reinnervation of transplanted hearts has also been derived from radionuclide studies using catecholamine analogs such as MIBG or HED. Because of limitations in spatial resolution, only the left ventricular tracer distribution can be adequately imaged by scintigraphy, not the atria or right ventricle. Therefore, one cannot draw conclusions directly from scintigraphic images regarding sinus node innervation. Patients who underwent cardiac transplantation more than 2 y before imaging revealed heterogeneous HED retention with increased uptake in the proximal anterior and septal walls (9). Our previous 11C-HED PET study after transplantation showed a continuous growth of sympathetic reinnervation once the reinnervation process was initiated (12). Anterobasal MIBG uptake indicating partial sympathetic reinnervation could be shown in 40% of the scintigraphically investigated patients 37–69 mo after transplantation (10). According to another group, patients studied from 2 to 12 y after transplantation had a heart-to-mediastinum ratio of MIBG significantly higher than patients studied <2 y after transplantation (13).
Applying qualitative scintigraphic analysis, a previous study by De Marco et al. (11) showed that MIBG uptake was observed in all 10 of the study patients after transplantation with positive transmyocardial NE release under resting conditions. No MIBG uptake was seen in any of the 6 study patients with negative release. However, they did not use tyramine injection to stimulate cardiac NE release. Their range of reinnervated NE release after transplantation was lower than the normal threshold (3 SD below mean normal value) as observed by another institute (4,5). These results suggest that rest NE release might fail to reflect the progress of heart reinnervation.
This study showed, for the first time, a close agreement between noninvasive quantitative PET imaging and tyramine-induced NE release measured by coronary catheterization. The quantitative analysis showed good correlation between tyramine-induced NE release and intensity and extent of HED retention of the transplanted heart. Two of 17 patients showed disagreement between scintigraphic results and NE release. The 2 patients also showed NE release from their hearts after tyramine injection. If the threshold of reinnervation had decreased to 109 pg/mL (2 SD), agreement would be 100% (φ coefficient = 1). However, for analysis, the previously validated threshold of 3 SD of baseline NE release was chosen. One explanation for the 2 disagreements could be a mechanism of the action of tyramine, which directly displaces NE from secretory granules in the nerve terminal. Compared with the HED results, tyramine-induced NE release may slightly underestimate reinnervation by ignoring sympathetic nerve terminals that contain only small or no functioning granules (25). In addition, negative values of ΔNECS–AO were seen in this study. On the technical point, some right atrial blood may contaminate the sample from the coronary sinus because of the placement of the coronary sinus catheter. However, our threshold of NE release calculated at baseline ΔNECS–AO was quite similar to that of previous studies. Wilson et al. (4,5) always took a 3 SD change of the baseline ΔNECS–AO of the patients as a threshold of reinnervation. This study’s threshold of 163 pg/mL lay between 178 pg/mL and 143 pg/mL, the reinnervation thresholds of the first and second studies, respectively. However, this study also used a threshold that was >7%/min, but that was still lower than reported normal thresholds of 8%–10%/min (12). The threshold may be more sensitive to subtle reinnervation than tyramine because HED retention is significantly correlated to the density of uptake-1 sites in the human heart (26).
Regional analysis of PET revealed no reinnervation in the inferior area up to 13 y after transplantation. Reinnervation may initiate along the large arteries (pulmonary artery and aorta) and slowly progress from the base to the apex of the left ventricle, as shown in animals (27). However, this regional process remains incomplete and surgical techniques may limit the growth of fibers across anastomoses, causing heterogeneous structural reinnervation. Close agreement between the scintigraphic results and NE release after tyramine injection confirms not only the specificity of the tracer approach for neuronal tissue, but also the recurrence of catecholamine uptake sites and tyramine-sensitive NE release from storage vesicles as a sign of normally responding sympathetic nerve terminals. The efficacy of a noninvasive scintigraphic method with HED PET for reinnervation after transplantation was validated in this study.
Although cardiac transplantation has been shown to considerably improve survival of patients with end stage heart failure, recovery of exercise tolerance remains unsatisfactory (28). Invasively measured tyramine-induced NE release in late reinnervated heart transplant recipients was associated with a positive inotropic and a coronary vasoconstrictor effect, both of which amounted to approximately 50% of that in healthy volunteers (6). Di Carli et al. (14) demonstrated that increases in coronary blood flow in response to sympathetic stimulation correlated with the regional NE content in the cardiac sympathetic-nerve terminals. These data suggest a beneficial effect of reinnervation in exercise performance and emphasize the need to optimize reinnervation in the human transplanted heart. Therefore, the scintigraphic approach will offer noninvasive repeat studies and regional assessment to define the influence of reinnervation on myocardial function.
This study’s HED negative group included more ischemic cardiomyopathy than the HED positive group, despite no significant difference. These results were different from previous studies, which reported that impaired reinnervation is more frequent in patients with dilated cardiomyopathy (11). Furthermore, rejection episodes may result in delay or suppression of the heart reinnervation, as suggested by a trend in this study. This study, however, focused on comparing HED retention with NE release and included a relatively small number of patients. Another, larger study including 35 patients with no significant difference of the number of rejection episodes or underlying disease between reinnervated and denervated groups (29) may be more reliable. Furthermore, mean NE release after tyramine injection in this study was less than Wilson’s mean values of approximately 486 pg/mL. Likelihood and magnitude of cardiac NE release, however, increased with the time interval between the transplantation and follow-up study (4), which may explain this difference.
CONCLUSION
Noninvasive neuronal imaging with 11C-HED PET was well correlated with invasively measured tyramine-induced NE release. The study further supports the usefulness of PET as a noninvasive approach for detection of the reappearance of catecholamine uptake sites after heart transplantation. HED not only reflects the reappearance of presynaptic uptake-1, but also identifies the presence of pharmacologically active nerve vesicles.
Acknowledgments
The authors thank the technologists and nurses in the PET suite and the clinic for heart surgery for their dedication and excellent technical assistance. The authors also thank the Department of Clinical Chemistry of the Technische Universität München for the careful measurement of NE. This study was supported in part by National Institutes of Health grant ROIHL47543.
Footnotes
Received Oct. 24, 2000; revision accepted Mar. 5, 2001.
For correspondence or reprints contact: Markus Schwaiger, MD, Nuklearmedizinische Klinik der TU München, Klinikum rechts der Isar, Ismaninger Str. 22, 81675 Munich, Germany.