Abstract
In vitro studies have shown that 99mTc-sestamibi (MIBI) is a transport substrate for the P-glycoprotein (Pgp) pump and the multidrug resistance–associated protein (MRP) pump. However, whether MRP and lung resistance protein (LRP) affect tumor accumulation and efflux of 99mTc-MIBI in lung cancer is not known. In this study, we explored whether Pgp and the other pumps, MRP and LRP, affect tumor accumulation and efflux of 99mTc-MIBI in lung cancer. Methods: Thirty-four lung cancer patients who underwent surgery were examined. Before surgery, 99mTc-MIBI SPECT was performed 15 min and 180 min after injection, and early uptake, delayed uptake (L/Nd), and washout rate (L/Nwr) of 99mTc-MIBI were obtained. Pgp, MRP, and LRP expression were investigated by immunohistochemistry. The messenger RNA (mRNA) level of Pgp, MRP, and LRP was determined by real-time reverse-transcription polymerase chain reaction. The lung cancer 99mTc-MIBI images were correlated with protein and mRNA expression. Results: The mean L/Nd of the Pgp (−) group was significantly higher than that of the Pgp (++) group (P = 0.0324). The Pgp (++) group had a higher L/Nwr than did the Pgp (−) group (P = 0.0269). The mean L/Nd of the Pgp mRNA low-expression group was significantly higher than that of the Pgp mRNA high-expression group (P = 0.0127). The Pgp mRNA high-expression group had a higher L/Nwr than did the Pgp mRNA low-expression group (P = 0.0825). No appreciable correlation was found between the lung cancer 99mTc-MIBI images and the expression of MRP or LRP on the level of protein or mRNA. Conclusion: These data suggest that an increased level of Pgp expression correlates with a low accumulation on delayed scans and a high L/Nwr of 99mTc-MIBI in lung cancer. Neither MRP nor LRP expression on the level of either protein or mRNA correlated significantly with tumor accumulation or efflux of 99mTc-MIBI in lung cancer.
- 99mTc-methoxyisobutylisonitrile
- lung cancer
- P-glycoprotein
- multidrug resistance–associated protein
- lung resistance protein
Multidrug resistance (MDR) is the main barrier to efficient chemotherapy of cancer. MDR has been closely associated with altered expression of MDR phenotype, and in lung cancer the MDR phenotype has been defined on the basis of the cellular drug targets involved, that is, P-glycoprotein (Pgp), MDR-associated protein (MRP), lung resistance protein (LRP), and atypical MDR (mediated through altered expression of topoisomerase type II) (1–4). In contrast to other types of MDR, pure atypical MDR is not associated with alterations in drug accumulation or retention (5). Pgp, encoded by the MDR1 gene, is a 170-kDa transmembrane glycoprotein and acts as an adenosine triphosphate (ATP)–driven drug efflux pump to reduce drug accumulation (6). MRP is a 190-kDa membrane-bound glycoprotein and can act as a glutathione S-conjugate efflux pump by transporting drugs that are conjugated or cotransported with glutathione (7,8). Both Pgp and MRP are integral membrane proteins belonging to the ATP-binding cassette (ABC) superfamily of transporter proteins, which appear to confer resistance by decreasing intracellular drug accumulation and enhancing drug efflux (9,10). In contrast, LRP is not an ABC transporter protein. LRP has recently been identified as a vault protein, which is a typical multisubunit structure involved in nucleocytoplasmic transport (11). Determination of these MDR proteins at the time of diagnosis is imperative to the development of rational therapeutic strategies for preventing drug resistance.
Nuclear medicine techniques, such as SPECT, that rely on biochemical and physiologic characteristics of the tumor have been used to evaluate the MDR phenotype. 99mTc-sestamibi (MIBI), a member of the isonitrile class of coordination compounds (12), is a lipophilic cation approved for clinical use as a myocardial perfusion imaging agent (13). 99mTc-MIBI has been reported to be useful for the detection of a variety of tumors, including lung cancer (14–17). 99mTc-MIBI, in addition to other drugs, has also been reported to be washed out from cells by a Pgp efflux pump (18). The potential advantage of 99mTc-MIBI imaging lies in its superiority in noninvasively detecting the presence of Pgp overexpression in vivo (19–21). Recently, 99mTc-MIBI efflux was shown to be a substrate for MRP in vitro (22). However, whether MRP and LRP affect tumor accumulation and efflux of 99mTc-MIBI in lung cancer is not known. The aim of this study was to determine whether Pgp and the other pumps, MRP and LRP, affect tumor accumulation and efflux of 99mTc-MIBI in lung cancer.
MATERIALS AND METHODS
Patients
Thirty-four patients (8 women, 26 men; age range, 37–82 y; mean age ± SD, 69.1 ± 8.9 y) with histologically confirmed lung cancer were included in the study. Twenty-two patients had adenocarcinoma, 5 had squamous cell carcinoma, 2 had small cell carcinoma, 2 had adenosquamous cell carcinoma, 1 had large cell carcinoma, and 2 had metastatic lung tumors. None had received prior treatment, including chemotherapy, radiotherapy, and surgery. All patients underwent 99mTc-MIBI SPECT followed by surgery. All tumor samples were analyzed by immunohistochemistry, and 10 samples were analyzed by reverse-transcription (RT) polymerase chain reaction (PCR).
99mTc-MIBI Chest Imaging
Chest imaging was performed with a double-head gamma camera equipped with a high-resolution parallel-hole collimator (PRISM 2000; Marconi Medical Systems, Cleveland, OH). Images were obtained 15 and 180 min after the injection of 740 MBq 99mTc-MIBI. Early and delayed SPECT of the chest was performed on all patients. For SPECT of the chest, 72 projections were obtained using a 64 × 64 matrix at 45 s per view. Image reconstruction was performed using filtered backprojection with Butterworth and ramp filters. Transverse, coronal, and sagittal sections were reconstructed. Attenuation correction was not applied.
SPECT images were compared with chest CT images, and accumulation in lung tumors was interpreted by 2 nuclear medicine physicians. The findings on the 99mTc-MIBI chest images were evaluated semiquantitatively. Regions of interest (ROIs) were manually defined on the transaxial tomograms that showed the lesion’s highest uptake to be in the middle of the tumor. The ROIs placed on the lesions (L) encompassed all pixels that had uptake values of >90% of the maximum uptake in that slice, and the average counting rate in each ROI was calculated. Another ROI of the same size was then drawn over the contralateral normal lung (N) on the same transverse section. The early uptake (L/Ne) and the delayed uptake (L/Nd) were obtained. The washout rate (L/Nwr) was calculated using the following formula: L/Nwr = (L/Ne − L/Nd) × 100/(L/Ne).
Immunohistochemistry
Expression of Pgp, MRP, and LRP was assessed by immunohistochemistry. The surgically resected tumors were routinely fixed in 10% formalin and embedded in paraffin. The largest section in the middle of the tumor was stained for immunohistochemical study, which was performed using a streptavidin–biotin complex. For immunostaining, the primary antibodies used were the mouse monoclonal antibody (mAb) against Pgp (JSB-1; Novocastra Laboratories Ltd., Newcastle, U.K.), the rabbit mAb against MRP (MRPr1; Nichirei Corp., Tokyo, Japan), and the mouse mAb against LRP (LRP-56; Nichirei Corp.). Sections 4 μm thick were cut from the formalin-fixed paraffin-embedded materials and treated with xylene to remove the wax. The endogenous peroxidase activity was blocked by incubation in 0.3% hydrogen peroxide in methanol. Nonspecific protein binding was inhibited by treatment with 10% normal serum for 10 min at 37°C, and the specimens were then incubated with JSB-1 (1:20), MRPr1 (1:10), or LRP-56 (1:10) overnight at 4°C. The slides were preincubated and rinsed in phosphate-buffered saline and then treated with the biotinylated secondary antibody and peroxidase-conjugated streptavidin. The final reaction product was revealed by exposure to 0.03% diaminobenzidine, and the nuclei were counterstained with Mayer’s hematoxylin. For a negative control, appropriately diluted nonimmune sera were applied instead of the primary antibodies. Kidney tissue (proximal tubules) from a healthy adult human was used as a positive control for Pgp (23), and colon tissue (epithelium) from a healthy adult human was used as a positive control for both MRP and LRP (24,25). The results of immunostaining were independently interpreted by a pathologist who knew none of the clinical information. Immunostaining was examined in 10 high-power fields. The average percentage of the positive area was calculated for 10 fields for each of the tumors. Pgp, MRP, and LRP expression was graded according to the percentage of stained tumor cells as − (0%–9%), + (10%–69%), or ++ (70%–100%).
Quantitative RT PCR
Standard Preparation.
The surgically resected tumor specimens were rapidly frozen in liquid nitrogen and stored at −70°C until processing. MDR1, MRP, and LRP transcripts were amplified by RT PCR and subcloned from patients known to carry the respective breakpoint. The cloned products were purified, and the identities of inserts were sequenced with ABI PRISM BigDye Terminators Cycle Sequencing Kit (a fluorescent sequencing ready reaction kit; Applied Biosystems, Foster City, CA). Plasmid DNA was serially diluted and ranged from 10:6 to 10:1 pg/μL. The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) standard was provided in the TaqMan GAPDH Control Reagents kit (Applied Biosystems). GAPDH messenger RNA (mRNA) serial dilutions ranged from 80 to 50 ng/mL
Real-Time RT PCR.
Expression of the target genes (MDR1, MRP, and LRP) and the endogenous reference GAPDH was quantified using the primers, probes, and standards. The primers and TaqMan probes were designed using the software Primer Express (Applied Biosystems) (Table 1). The RT PCR was performed according to a TaqMan 2-step method using an ABI PRISM 7700 Sequence Detection System (Applied Biosystems). The nontemplate controls, standard dilutions, and tumor samples were assayed. Total RNA was prepared from the frozen specimens. Total RNA (200 ng) isolated from tumor samples was reverse transcribed to complementary DNA (cDNA) using an oligo deoxythymidine primer. A 25-mm volume of PCR reaction mixture was used, containing 200 ng of the sample cDNA; TaqMan buffer; 200 mmol/L deoxy-ATP, deoxycytidine triphosphate, and deoxyguanosine triphosphate; 400 mmol/L deoxyuridine triphosphate; 5.5 mmol/L magnesium chloride; 0.025 U/mL AmpliTaq Gold DNA polymerase (Applied Biosystems); 0.01 U/mL AmpErase uracil N-glycosylase (Applied Biosystems); 200 nmol/L forward and reverse primers; and 100 nmol/L probe. The PCR cycling conditions included an initial phase of 2 min at 50°C, followed by 10 min at 95°C for AmpErase, 40 cycles of 15 s at 95°C, and 1 min at 60°C.
Oligonucleotides Used for PCR Amplifications
Quantification of the PCR products was based on the TaqMan 5′ nuclease assay (26) using the ABI PRISM 7700 Sequence Detection System. The starting quantity of a specific mRNA in an unknown sample was determined by preparing a standard curve using known dilutions of the standard cDNA. The standard curve was generated on the basis of the linear relationship between the Ct value (corresponding to the cycle number at which a significant increase in the fluorescence signal was first detected) and the logarithm of the starting quantity (27). The unknown samples were quantified by the software of the ABI PRISM 7700 Sequence Detector System, which calculated the Ct value for each sample and then determined the initial quantity of the target using the standard curve. The amount of the target was normalized to the GAPDH reference. In accord with our results for agarose electrophoresis, the limit for Pgp mRNA high expression was set at Pgp/GAPDH > 0.03, the limit for MRP mRNA high expression was set at MRP/GAPDH > 0.03, and the limit for LRP mRNA high expression was set at LRP/GAPDH > 0.1.
Statistical Analysis.
The results for L/Ne, L/Nd, and L/Nwr were expressed as mean ± 1 SD. The differences in L/Ne, L/Nd, and L/Nwr between patients with (−), (+), and (++) Pgp, MRP, or LRP expression were determined using an independent Student t test. The differences in L/Ne, L/Nd, and L/Nwr between patients with high and low Pgp mRNA, MRP mRNA, or LRP mRNA expression were determined using an independent Student t test. If P was <0.05, the difference was considered significant.
RESULTS
All 34 surgically obtained tissue samples were assessed to estimate the levels of Pgp, MRP, and LRP expression on protein level. Ten samples were analyzed to estimate the levels of Pgp, MRP, and LRP expression on mRNA level. Table 2 summarizes the immunohistochemical results and the RT PCR data.
Patient Characteristics and Radionuclide Imaging Results
Correlation of 99mTc-MIBI Results with Immunohistochemical Results
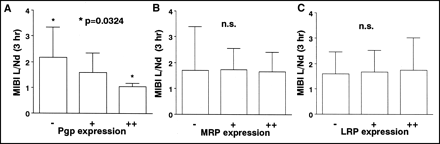
No correlation was found between L/Ne and the expression of Pgp, MRP, or LRP. The mean L/Nd (2.182 ± 1.165) of the Pgp (−) group was significantly higher than that (1.058 ± 0.143) of the Pgp (++) group (P = 0.0324) (Figs. 1, 2, and 3A). L/Nd was not related to the expression of MRP or LRP (Figs. 3B and 3C). The Pgp (++) group had a higher mean L/Nwr (40.68% ± 21.657%) than did the Pgp (−) group (−0.66% ± 35.066%, P = 0.0269) (Figs. 1, 2, and 4A). L/Nwr was not related to the expression of MRP or LRP (Figs. 4B and 4C).
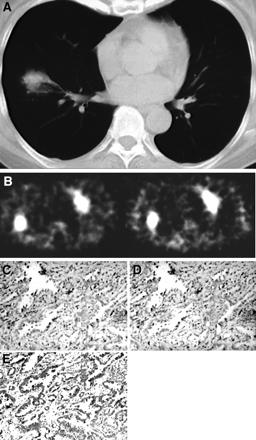
Patient 1, 73-y-old man with 2.8 × 2.8 cm lung adenocarcinoma. (A) CT scan shows nodule in right lung. (B) Early 99mTc-MIBI SPECT scan (left) shows intense uptake (arrow) of 99mTc-MIBI in tumor (L/Ne = 3.81). Delayed scan (right) shows faint 99mTc-MIBI uptake in tumor (L/Nd = 1.00), suggesting rapid washout of 99mTc-MIBI from tumor (L/Nwr = 73.8%). Immunohistochemistry (×200) reveals strong Pgp expression (C), strong MRP expression (D), and weak LRP expression (E) in corresponding tumor tissue.
Patient 34, 68-y-old woman with 3.6 × 3.6 cm lung adenocarcinoma. (A) CT scan shows nodule in right lung. (B) Early 99mTc-MIBI SPECT scan (left) shows intense uptake of 99mTc-MIBI in tumor (L/Ne = 3.65). Delayed scan (right) also shows intense 99mTc-MIBI uptake in tumor (L/Nd = 5.17), suggesting slow washout of 99mTc-MIBI from tumor (L/Nwr = −41.6%). Immunohistochemistry (×200) reveals negative Pgp expression (C), negative MRP expression (D), and strong LRP expression (E) in corresponding tumor tissue.
Relationship between expression of Pgp, MRP, or LRP and 99mTc-MIBI L/Nd in lung cancer. (A) L/Nd correlated inversely with density of Pgp expression. Statistically significant difference in L/Nd was found between Pgp (−) group and Pgp (++) group. (B) No significant difference (n.s.) in L/Nd was found among MRP (++), MRP (+), and MRP (−) groups. (C) No significant difference in L/Nd was found among LRP (++), LRP (+), and LRP (−) groups.
Relationship between expression of Pgp, MRP, or LRP and 99mTc-MIBI L/Nwr in lung cancer. (A) L/Nwr correlated with density of Pgp expression. Statistically significant difference in L/Nwr was found between Pgp (++) group and Pgp (−) group. (B) No significant difference (n.s.) in L/Nwr was found among MRP(++), MRP (+), and MRP (−) groups. (C) No significant difference in L/Nwr was found among LRP (++), LRP (+), and LRP (−) groups.
Correlation of 99mTc-MIBI Results with RT PCR Results
No correlation was found between L/Ne and the level of Pgp mRNA, MRP mRNA, or LRP mRNA. The mean L/Nd (2.57 ± 0.731) of the Pgp mRNA low-expression group was significantly higher than that (1.318 ± 0.519) of the Pgp mRNA high-expression group (P = 0.0127) (Fig. 5A). L/Nd was not related to the level of MRP mRNA or LRP mRNA. Statistical support was found toward a significant difference in L/Nwr between the Pgp mRNA high-expression group (31.967% ± 44.447%) and the Pgp mRNA low-expression group (−21.450% ± 25.651%, P = 0.0825) (Fig. 5B). L/Nwr was not related to the level of MRP mRNA or LRP mRNA.
Relationship between Pgp mRNA expression and 99mTc-MIBI L/Nd or 99mTc-MIBI L/Nwr in lung cancer. (A) L/Nd correlated inversely with Pgp mRNA level. Statistically significant difference in L/Nd was found between Pgp mRNA low-expression group and Pgp mRNA high-expression group. (B) Statistical support was found toward a significant difference in L/Nwr between Pgp mRNA high-expression group and Pgp mRNA low-expression group.
DISCUSSION
Evidence has shown that Pgp as a drug efflux pump extrudes 99mTc-MIBI and other drugs from cells and that Pgp expression and enhanced efflux of 99mTc-MIBI from these cells are closely connected (18,22). In animal models, faster clearance of 99mTc-MIBI is observed in tumors that express Pgp than in those that do not express Pgp (3,18). There have also been some reports about the clinical relevance of Pgp and 99mTc-MIBI. A study of untreated breast cancer patients showed that 99mTc-MIBI L/Nwr values from breast cancers overexpressing Pgp were 2.7 times higher than those from cancers not expressing Pgp (21). An inverse correlation between the tumor-to-background ratio of 99mTc-MIBI and the density of Pgp expression in the tumors of patients with breast and lung cancer has also been reported (19). Our clinical data revealed that the mean L/Nd of the Pgp (−) group was significantly higher than that of the Pgp (++) group (P = 0.0324) (Figs. 1, 2, and 3A). The Pgp (++) group had a higher L/Nwr than did the Pgp (−) group (P = 0.0269) (Figs. 1, 2, and 4A). No correlation was found between L/Ne and Pgp expression. The same results were obtained for mRNA level. The mean L/Nd of the Pgp mRNA low-expression group was significantly higher than that of the Pgp mRNA high-expression group (P = 0.0127) (Fig. 5A). The Pgp mRNA high-expression group had a higher L/Nwr than did the Pgp mRNA low-expression group (P = 0.0825) (Fig. 5B). No correlation was found between L/Ne and Pgp mRNA level. Uptake of tumor 99mTc-MIBI is associated with many factors, including direct mechanisms such as negative transmembrane potential and drug efflux pump and indirect mechanisms such as blood flow and capillary permeability. We considered L/Ne to be more affected by blood flow. In contrast, L/Nd and L/Nwr clearly reflected Pgp expression of intrinsic properties of the tumor.
It has been established that MRP belongs to the superfamily of ABC transmembrane transporter proteins and can act as a glutathione S-conjugate efflux pump (7–9). Recently, 99mTc-MIBI was shown to be a substrate for MRP in vitro (22). The abilities of Pgp and MRP transporters to wash out 99mTc-MIBI have been reported to be similar in cell lines, in spite of different suspected mechanisms of transport (28). However, cardiac muscle showed a low L/Nwr of 99mTc-MIBI and a low level of Pgp expression but a high level of MRP expression (23,24). The efficiencies of Pgp and MRP in transporting 99mTc-MIBI seem to be different in vivo. Likewise, in our clinical data, we did not observe any correlation between tumor accumulation or efflux of 99mTc-MIBI and the expression of MRP on protein level or mRNA level in lung cancer. We suppose that the discrepancy between in vitro and in vivo findings is closely associated with glutathione. The exact way in which glutathione is involved in MRP-mediated transport is unclear. One possibility is that glutathione forms transportable complexes with cationic agents or reduces essential functional groups in the protein. Another possibility is that glutathione functions as a cotransporter to pump cationic drugs out of the cell by way of MRP (29). As observed in our data, these hypotheses suggest that glutathione and glutathione-S-transferase ([GST] an enzyme that catalyzes the glutathione conjugation reaction) are the essential functional components in glutathione-dependent MRP-mediated transport. Recently, depleted glutathione levels have been reported with reduced MRP-mediated MIBI transport (30). Increased glutathione levels have also been reported with resistance to alkylating agents and cisplatin (31). In addition, a significant correlation was found between resistance to doxorubicin and the expression of GST in 94 cases of non–small cell lung carcinoma (32). Therefore, although MIBI imaging of lung cancer is not associated with expression of MRP, MIBI imaging may be related to the levels of glutathione and GST, which reflect the functionality of MRP-mediated transport.
LRP has been identified as the vault protein involved in nucleocytoplasmic transport (11). Recently, subcellular accumulation of drugs was found to be localized in the cytoplasm and minimally in the nuclei in LRP overexpression cells (33). Because of previous findings that increased cytoplasm concentration of the drug increases its contact with the membrane, we believed that efflux of the drug may be enhanced in LRP overexpression cells. However, we did not find a correlation between tumor accumulation or efflux of 99mTc-MIBI and expression of LRP. Subcellular accumulation of 99mTc-MIBI within the mitochondria and cytoplasm of cells has been reported to be based on transmembrane electric potentials (34). Therefore, efflux of 99mTc-MIBI was rarely affected by expression of LRP.
This study had limitations. First, some samples showed a discordance between MDR protein and mRNA levels, whereas Pgp expression on protein level (percentage of stained tumor cells) correlated significantly with mRNA expression (r = 0.654; P = 0.0404). This phenomenon may be explained by the biologic heterogeneity of tumors. Intratumoral heterogeneity of histologic features is widely recognized, having been most extensively studied in pulmonary carcinoma (35). In our study, the largest section in the middle of the tumor was stained for immunohistochemistry, because ROIs on 99mTc-MIBI SPECT were defined on the transaxial tomograms that showed the lesion’s highest uptake to be in the middle of the tumor. RNA was prepared from a different area near the middle of the tumor. Second, the correlation of 99mTc-MIBI results with Pgp expression was obtained without taking into account MRP and LRP. Interaction between the 3 MDR proteins cannot be ruled out. Third, the results of this study address investigational more than clinical issues, because MDR is multifactorial. We did not calculate the correlation between response to chemotherapy and 99mTc-MIBI imaging because all patients underwent surgery. Furthermore, measurement of mRNA expression of MDR proteins does not necessarily provide information about the dynamic function of the drug efflux pump. MDR may be defined not by the number of MDR proteins on tumor cells but by the functional transport capacity of drug efflux pumps. Clearly, additional clinical study is essential.
CONCLUSION
In this preliminary study, we searched for correlations between both tumor uptake and clearance of 99mTc-MIBI with an MDR phenotype (Pgp, MRP, and LRP) on protein and mRNA levels. A close connection exists between 99mTc-MIBI imaging of lung cancer and expression of Pgp. The L/Nd and L/Nwr of 99mTc-MIBI are useful for noninvasively detecting the expression of Pgp in lung cancer. 99mTc-MIBI may not be useful in identifying the expression of MRP or LRP.
Acknowledgments
This study was supported by grants for project research (H00-2 and A99-1) from the High-Technology Center of Kanazawa Medical University; a grant-in-aid for cancer research (12-4) from the Ministry of Health and Welfare, Japan; and a grant-in-aid for scientific research (13670192) from the Ministry of Education, Science, and Culture, Japan.
Footnotes
Received Mar. 1, 2001; revision accepted Jun. 12, 2001.
For correspondence or reprints contact: Kotaro Higashi, MD, Department of Radiology, Kanazawa Medical University, 1-1, Daigaku, Uchinada, Kahoku-gun, Ishikawa, 920-0293, Japan.