Abstract
Accurate staging is elementary for optimal management of malignant lymphoma. Advanced cases may be curable with multidrug chemotherapy combined with radiotherapy, whereas limited disease can sometimes be cured by local radiotherapy only. Recently, FDG imaging with whole-body PET (WB PET) has been introduced as an accurate method for staging lymphoma. We evaluated the usefulness of l-[methyl-11C]methionine (MET) in comparison with FDG as a tracer for nodal staging of lymphoma with WB PET. Methods: Nineteen patients with untreated, histologically proven malignant lymphoma underwent WB PET imaging with MET and FDG within 1 wk before treatment. Fourteen patients had non-Hodgkin's lymphoma (NHL), and 5 had Hodgkin's disease (HD). Two of these 19 patients were excluded from the final analysis because of hyperglycemia. WB PET images using FDG and MET were visually compared by 3 independent interpreters, and the PET findings were correlated with the data on the basis of conventional staging studies. Results: Fifty-five of 178 lymph node regions were classified as diseased both by FDG PET and by CT, and 54 of 178 were classified as diseased both by MET PET and by CT. In addition, 11 lymph node regions that CT showed to be normal avidly accumulated FDG. Ten of these lymph node regions also had clear uptake of MET. Another 4 and 5 lymph node regions were enlarged at CT but were judged to be normal by FDG and MET PET, respectively. In nodal staging, both FDG PET and MET PET would have upstaged the disease in 3 patients. MET PET would also have downstaged the disease in 1 patient. Conclusion: FDG and MET seem to be comparable in the detection of lymphoma by WB PET. However, visual interpretation of the images tends to be hampered more by physiologic accumulations of MET than by normal accumulations of FDG, and MET may be preferable to FDG in hyperglycemic patients undergoing staging studies with PET.
Among the improvements in cancer management are impressive advances in the treatment of lymphoma. Most patients with Hodgkin's disease (HD) and non-Hodgkin's lymphoma (NHL) can be treated curatively. In addition to histologic type, several prognostic factors are considered in planning the treatment of patients with lymphoma. Of these, the extent of disease is elementary for optimal selection of treatment strategy. Patients presenting with local or locoregional disease are often best treated with a combination of moderately intensive chemotherapy and involved-field radiotherapy. On the other hand, patients with a histologically aggressive tumor and advanced disease are candidates for intensive high-dose chemotherapy schedules, often including bone marrow transplantation with autologous stem cell support.
The conventional staging methods routinely used in patients with HD and NHL include chest radiography; a complete blood count; CT of the neck, chest, abdomen, and pelvis; bone marrow aspiration and biopsy; and nasoendoscopy in addition to a careful clinical history and physical examination. Bipedal lymphangiography is still sometimes used in patients with HD, whereas the role of staging laparotomy is likely to decrease. The standard staging system of NHL and HD has been based on the Ann Arbor classification for more than 20 y (1). This staging system takes into account the extent of nodal involvement and extranodal manifestations and divides patients into 4 groups representing stages I–IV.
CT and MRI use morphologic criteria to detect disease. The diagnosis of lymph node abnormalities both with CT and with MRI largely depends on lymph node size. Lymph nodes that are smaller than 10 mm are usually regarded as disease free. However, normally sized lymph nodes may be diseased, and in contrast, enlarged lymph nodes may show an inflammatory response (for example) and be free of disease (2,3). 67Ga scintigraphy may be useful for detection of a posttreatment residual tumor mass but has not been shown to be superior to CT in initial staging of primary lymphoma (4,5).
PET offers the unique capability of revealing metabolic activity throughout the body. An important role of FDG as a tumor-seeking agent has been established for various malignant tumors (6,7). Increased glucose metabolism is a basic biochemical hallmark of neoplastic cells (8,9), and the glucose analog FDG is transported, phosphorylated, and metabolically trapped in the malignant cells. Both high- and low-grade lymphomas can be imaged well with FDG PET (10). Whole-body PET (WB PET) with FDG has recently been shown feasible for staging lymphoma (11–17).
l-[methyl-11C]methionine (MET) is another widely used tumor-seeking tracer for PET. Methionine is an essential amino acid needed for protein and polyamine synthesis and is also involved in transsulfuration and transamination reactions. Furthermore, methionine acts as a precursor for S-adenosyl-methionine, which is the predominant biologic methyl group donor (18). Methionine metabolism is altered in cancer cells, and the accumulation of MET in malignant tumors is principally caused by its enhanced transport across the plasma membrane of neoplastic cells (19). MET has been successfully used in PET studies on tumors of the human brain, breast, lung, and head and neck and on lymphoma (20–27).
Only a few studies on lymphoma detection by MET PET have been reported. Leskinen-Kallio et al. (25) studied 14 patients with NHL and found that MET uptake was increased both in high- and low-grade lymphoma, whereas FDG was found to be superior to MET in distinguishing high-grade tumors from the other grades. Rodriquez et al. (26) investigated 23 lymphoma patients and also concluded that FDG uptake was associated with malignancy grade, but no relationship was found between MET uptake and malignancy grade. In reporting a recent study, Nuutinen et al. (27) concluded that MET PET is highly sensitive for detection of untreated and recurrent lymphoma. Unlike the 2 earlier studies, this study found that differentiation of high-grade lymphomas from lower malignancy grades may be possible if graphic analysis is applied to calculate the influx constant for MET uptake. All these studies involved imaging of a single lymph node region, and no attempt was made to stage lymphoma with MET PET.
Furthermore, relatively few studies have compared uptake of FDG and MET in untreated tumors of the same patient. Although both FDG and MET are suited to detect lymphoma deposits, the different metabolic routes and physiologic biodistribution of these 2 tracers may provide a different clinical role in WB PET of lymphoma. In this study, we assessed the feasibility of MET as a tracer for WB PET and specifically compared the role of MET with that of FDG in detecting nodal disease in patients with newly diagnosed HD or NHL.
MATERIALS AND METHODS
Patients
Nineteen patients (11 men, 8 women; age range, 26–81 y; median age, 55 y) with newly diagnosed untreated lymphoma admitted to our institution between February 1997 and December 1997 were enrolled in the study. The lymphatic malignancy was histologically confirmed in all patients, of whom 5 had HD and 14 had NHL. All patients with HD had the nodular sclerosis type, and among patients with NHL, 10 had low-grade lymphomas, 2 had intermediate-grade lymphomas, and 2 had high-grade lymphomas. NHL was classified according to the updated Kiel classification (28) and the Working Formulation scheme (29). Clinical staging was done according to the Ann Arbor classification system. Routinely used conventional staging procedures included a careful physical examination; chest radiography; combinations of CT of the neck, chest, abdomen, and pelvis; indirect nasoendoscopy; total blood count; unilateral crest bone marrow aspiration and biopsy; and, in patients with HD, bipedal lymphangiography. Oral and written informed consent was obtained from each patient before enrollment. The study protocol was approved by the joint ethical committee of the University of Turku and Turku University Central Hospital.
All patients were advised to fast at least 6 h before the PET study, and their plasma glucose levels were monitored immediately before FDG injection. Seventeen patients had a normal plasma glucose concentration (median, 5.2 mmol/L; range, 4.2–5.9 mmol/L). Two patients had overt hyperglycemia (plasma glucose level, 13.2 and 16.5 mmol/L) and were excluded from the site-by-site analysis because of impaired FDG image quality caused by hyperglycemia (30).
PET Imaging
Both FDG and MET were synthesized at the Radiopharmaceutical Chemistry Laboratory of Turku PET Center. FDG synthesis was a modification of the method reported by Hamacher et al. (31). MET was synthesized from [11C]methyl triflate as described earlier (32). The radiochemical purity of both tracers always exceeded 98%.
PET studies were performed with an Advance PET scanner (General Electric Medical Systems, Milwaukee, WI) operated in 2-dimensional mode. The scanner consists of 18 rings of bismuth germanate detectors yielding 35 transverse slices spaced by 4.25 mm. The imaging field of view is 55 cm in diameter and 15.2 cm in axial length (33).
WB PET imaging with MET and FDG was performed in random order within 1 wk before initiation of treatment. A median dose of 439 MBq MET (range, 321–478 MBq) and 370 MBq FDG (range, 292–395 MBq) was injected into a peripheral vein of the upper extremity. After the injection, the patients were asked to stay at rest and to void just before FDG scanning. No transmission scans were obtained. Emission scanning was started 15 min after the injection of MET and 30 min after the injection of FDG. The imaging was initiated at the upper ridge of the orbita and ended at the upper thigh. Six or 7 bed positions (90–105 cm) were required for this procedure, with an acquisition time of 5 min per position. Multiple laser-guided landmarks were used for exact repositioning between FDG PET and MET PET studies. The acquired data were reconstructed without attenuation correction into (128 × 128) transaxial images using a standard filtered backprojection technique and Hanning filter. The transaxial images were then resliced into tomographic coronal and sagittal views. The in-plane resolution (full width at half maximum) was 5 mm in the center of the field of view, and the axial resolution was 6 mm.
PET Analysis
Tomographic PET images in the transaxial, coronal, and sagittal planes were visually analyzed on a high-resolution display monitor (Sun workstation; Sun Microsystems, Inc., Palo Alto, CA). PET images were visually interpreted independently by 3 investigators unaware of the data from conventional staging studies. For the analysis of lesion detectability in particular lymph node regions, the WB PET findings were tabulated into anatomic sites as follows: left and right cervical, left and right supraclavicular, left and right axillary, mediastinal or hilar, para-aortal, mesenteric, para-iliac, and left and right inguinal.
Any foci of tracer uptake that were clearly detectable against a normal background and were not in areas of known physiologically increased uptake were considered to be suggestive of lymphoma. The visual uptake of tracer at a specified lymph node region was scored as positive or negative for lymphoma. When regions were classified as positive or negative, the location, size, shape, and lateral asymmetry of the suspected lesions were also considered. Increased focal or inhomogeneous uptake patterns were considered suggestive of lymphoma in the organs with physiologic tracer uptake (e.g., bone marrow and spleen). In cases of interobserver discrepancy, the 3 investigators reached the final judgment by consensus.
In the final analysis, only regions imaged with both CT and PET were included. If both CT and PET findings agreed on the status of a lymph node region or organ, it was regarded as truly diseased or truly free of disease. Follow-up, evaluation of response to treatment, or biopsy was used for confirmation in cases of discrepant lesions. Of the patients with discrepant lesions, 2 underwent follow-up PET; 5, CT; and 1, sonography. Any lesion seen to have clearly responded to subsequent chemotherapy during the follow-up examination was regarded as diseased. In most cases, systematic biopsies of various lesions were not obtained for ethical reasons.
CT
CT scans were obtained within a median of 10 d from the 2 PET studies and were performed mainly with a Pace CT scanner (General Electric Medical Systems) or a PQ 2000 CT scanner (Picker International, Cleveland, OH) using standard methods for image acquisition. The studies of the neck, thorax, abdomen, and pelvis were performed before initiation of treatment, with a slice thickness of 5 mm in the neck and 10 mm in the thorax, abdomen, and pelvis. Intravenous contrast enhancement was used in all patients. Abdominal and pelvic CT scans were obtained with orally administered contrast material.
The CT images were interpreted by an experienced radiologist without knowledge of the PET findings or clinical data. The distribution of the lymph node regions was the same as for the PET analysis. Lymph nodes more than 10 mm in axial diameter were considered malignant. Lymph nodes were also considered malignant if multiple nodes smaller than 10 mm in axial diameter were seen in a single region or if a central low-density area suggested necrosis. Enlarged lymph nodes and nodal masses seen on CT scans were measured in 2 or 3 dimensions. The spleen and liver were regarded as malignant if they were massively enlarged or contained suggestive infiltrates.
RESULTS
Detection of Nodal Disease
Excluding the 2 patients with diabetes, a total of 178 lymph node regions in 17 patients were imaged with both CT and PET. Of these 178 regions, 66 showed increased FDG uptake and 64 showed increased MET uptake. Fifty-five of the 66 regions with abnormal findings on FDG PET were interpreted as pathologic on CT also (83%). Fifty-four of the 64 nodal regions with abnormal MET uptake were interpreted as pathologic on CT also (84%).
Another 4 lymph node regions in 4 patients were enlarged only on CT, and no abnormal accumulation of tracer was seen in these regions on the FDG images. Five regions in 5 patients were found to have enlarged lymph nodes on CT, whereas no uptake of MET could be seen on PET in the corresponding areas. All 4 FDG-negative but CT-positive lymph node areas were included in these 5 MET-negative regions. Eleven lymph node regions in 9 patients had increased uptake on FDG PET, although CT had shown normal findings, and 10 of the same regions in 8 patients had increased uptake on MET PET (Tables 1 and 2).
PET and CT Findings in Involved Lymph Node Regions in 17 Patients with Lymphoma
Manifestation of Discrepant Lesions in Lymph Node Regions
PET-Positive and CT-Negative Nodal Regions
Eleven discrepant lymph node regions suggestive of lymphoma were found on FDG PET, and 10 were found on MET PET. Interestingly, these 10 areas on MET PET were the same found to be discrepant on FDG PET. Two of these discrepant FDG PET lesions were regarded as true-positive, and the same 2 were true-positive on MET PET. One of these 2 was a supraclavicular lymph node that was retrospectively regarded as malignant on CT also and became smaller during therapy (patient 10). Another was an inguinal lymph node that also became smaller during therapy and was regarded as malignant (patient 6).
One of 11 discrepant FDG PET lesions was regarded as false-positive. This nodular-shaped FDG-positive lesion at the right parailiac site disappeared during the 14-mo follow-up without treatment. The patient (patient 1), who, despite his high-grade lymphoma, refused all treatment, had an endovascular prosthesis because of an operation for aneurysmal aorta 2 mo before the PET study. Obviously, the abnormal accumulation of FDG was caused by an inflammatory process around the prosthesis shortly after the operation. In this patient, MET PET was correctly negative in the right parailiac area. Two other discrepant PET findings with both FDG and MET were left and right inguinal nodes in patient 11, who had low-grade angioimmunoblastic T cell lymphoma. Although this patient received no treatment, the inguinal PET-positive lesions disappeared on a follow-up PET scan. The discrepant enlarged para-aortal lymph node seen on CT but not on PET of the same patient also disappeared on follow-up CT. Although lesions of this kind of low-grade lymphoma can spontaneously disappear, we regarded these discrepant findings as unresolved. The remaining 6 discrepant lesions in 4 patients also could not be resolved (Table 2).
CT-Positive and PET-Negative Nodal Regions
CT showed 5 additional suggestive areas when compared with MET and 4 discrepant areas when compared with FDG PET. These 4 negative areas on FDG PET were the same as the areas that were negative on MET PET. Two of these discrepant CT findings were regarded as true-positive on follow-up CT in comparison both with MET and with FDG PET. In both of these patients, the discrepant lesions were para-aortic lymph nodes. Patient 4 had multiple small para-aortic lymph nodes (0.5–1.5 cm in diameter), which became smaller during chemotherapy. Patient 15 had 2 involved para-aortic lymph nodes, which were 1.5 × 1.5 and 1.0 × 1.0 cm and also became smaller during chemotherapy. In comparison with MET PET, 1 more true-positive CT finding was encountered. This patient had 0.5- to 1.5-cm para-aortic lymph nodes, which appeared smaller on follow-up CT during therapy.
One false-positive CT lesion had no uptake on either FDG or MET PET. In patient 1, an inguinal lymph node (1.5 × 1.5 × 1.5 cm) showed only normal lymphocytes at fine-needle biopsy and was regarded as showing false-positive CT findings. One of the discrepant CT lesions remained unresolved. This was a para-aortic lesion (1.5 × 1.5 × 4 cm) that had disappeared on follow-up CT without any therapy. The patient (patient 11) had angioimmunoblastic low-grade lymphoma.
Detection of Extranodal Disease
Extranodal lesions were detected in 5 of 17 patients by conventional methods. All 5 patients had histologically verified bone marrow involvement. In addition, 2 of these 5 patients had involvement of the spleen, as shown by massive enlargement of the organ on CT. FDG PET was concordant with conventional methods in 2 patients and with MET PET in 1 patient in revealing bone marrow involvement. In 1 patient, FDG and MET PET were concordant with CT in revealing involvement of the spleen, whereas in the other patient in whom CT revealed spleen involvement, FDG and MET did not reveal the involvement.
Staging Comparison
Nodal staging based on FDG PET would have resulted in restaging of only 3 patients if all FDG PET lesions had been considered. The stage of 2 patients would have been changed from II to III, and that of a third patient would have been changed from I to II. Interestingly, MET PET findings would have upstaged the disease in the same 3 patients. MET PET would also have downstaged the disease from III to II in 1 patient (patient 16) with enlarged para-aortal lesions on CT but no abnormal MET uptake in the corresponding region. In 1 patient (patient 1), MET also correctly showed stage I disease when CT showed a false-positive inguinal lesion and FDG showed a false-positive para-aortal lesion, incorrectly leading to stage II disease. Considering the treatment policy at our institution, the changes in nodal staging would have modified treatment in only 1 patient (patient 5) similarly with both tracers. If extranodal lesions had also been considered, FDG PET would have upstaged the disease in 2 patients and downstaged the disease in 3 patients because of failure to detect bone marrow involvement. MET PET would have upstaged the disease in the same 2 patients and downstaged the disease in 5 patients, 3 of whom were the same as those who would have been downstaged with FDG PET.
Table 3 shows the nodal stages obtained from conventional imaging and PET studies in individual patients. When extranodal lesions were added to final staging, 5 patients were found to have stage IV disease. All of these 5 (patients 2, 3, 9, 10, and 17) had bone marrow infiltration.
Patient Characteristics and Stage in 17 Patients with Lymphoma
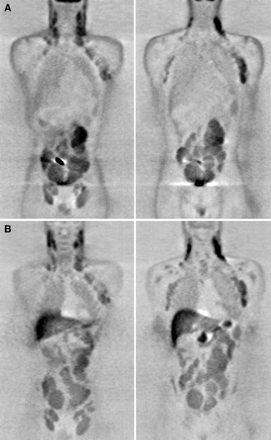
Generally, FDG and MET seemed to accumulate avidly in both low- and high-grade tumors, and no distinct difference in the pattern of lesion visualization could be shown among the different histologic subtypes. Of 2 hyperglycemic patients excluded from site-by-site analysis, 1 had HD with newly diagnosed diabetes and the other had low-grade NHL. In the former, the lymphomatous deposits in the mediastinum were not seen at FDG PET, but MET clearly showed all suggestive lesions of the mediastinum, right axilla, and sternum that were detected on CT (Fig. 1). Another hyperglycemic patient had stage I disease on the right side of the neck. Both FDG and MET PET showed focal uptake, although MET PET showed it more clearly.
WB PET images of 26-y-old man with stage IV HD (nodular sclerosis type) and untreated diabetes mellitus (blood glucose, 16.5 mmol/L at time of PET scanning). MET PET image (A) shows mediastinal lesions (arrow) that are not seen on FDG PET image (B). MET findings were also accordant with CT lesions in right axilla and distal part of sternum, which are not depicted in this coronal plane.
DISCUSSION
Nodal staging is instrumental but not the only factor defining the treatment strategy for a patient with HD or NHL. The histologic type of lymphoma, presence of B-symptoms, age, serum lactate dehydrogenase content, extent of disease, and extranodal manifestations are also important prognostic factors and used in selecting the appropriate treatment modality. However, nodal staging is the major factor differentiating limited from advanced disease. Currently, advanced disease is best managed by aggressive chemotherapy schedules leading to a variety of side effects, such as nausea, fatigue, alopecia, neuropathy, neutropenia, and infections. However, involved field radiotherapy, possibly combined with a short course of chemotherapy, can usually be performed with fewer adverse effects and is often curative for limited disease. Hence, the importance of accurate staging cannot be overestimated in the management of lymphoma.
Conventional staging procedures consist of morphologic imaging by CT, bone marrow biopsy, and sometimes laparotomy. Sonography can also be used, especially to guide biopsies, but the noninvasive assessment of lymph node pathology is still mostly based on size criteria. Usually, nodes smaller than 10 mm are considered to be free of disease. However, normal-sized nodes may contain neoplastic cells, whereas enlarged nodes may have normal histology and show only immunoreactive cells. Evaluation of lymphoma with MRI also depends on size criteria and has not replaced CT in the clinical setting.
This study was designed to compare MET with FDG as tracers for WB PET studies in the nodal staging of lymphoma. MET accumulates avidly in tumor tissues and has been reported to effectively image several cancer types. Indeed, accumulation of MET in malignant lymph nodes was comparable with that of FDG in this study (Figs. 2 and 3), and FDG and MET seemed to accumulate avidly in both high- and low-grade lymphomas. The total number of discrepant findings in individual lymph node sites (15/178) on CT and PET was similar with FDG and MET. However, the different biodistribution of the 2 tracers may interfere with interpretation of the scans in spite of the similar potential for nodal detection. As an example, the avid physiologic accumulation of MET in multiple abdominal organs (liver, pancreas, intestine, and bone marrow) concealed a group of pathologic lymph nodes detected by FDG and CT in 1 patient. On the other hand, MET was superior to FDG at detecting mediastinal disease in the patient with diabetes. The evaluation of extranodal disease in bone marrow and liver by MET PET seems to be difficult because of high physiologic accumulations of MET in normal organs (21). By contrast, successful detection of extranodal disease with FDG has been reported by many authors (15–17).
WB PET images of 51-y-old woman with stage IV low-grade NHL in whom CT, FDG, and MET PET were concordant in detection of nodal findings. In FDG images (A), 2 coronal slices show intense focal uptake in multiple lymph node regions both superior and inferior to diaphragm. (B) Coronal MET PET images show similarly intense focal uptake in multiple lymph node regions. Physiologic uptake pattern differs between FDG (e.g., urinary bladder) and MET (e.g., liver, pancreas).
Coronal PET images of 50-y-old man with low-grade NHL (stage IV). FDG (A) and MET (B) images show similarly enhanced uptake in cervical, supraclavicular, axillary, abdominal, and inguinal regions.
WB PET provides a metabolic way to image the entire body in a single session. WB PET with FDG has been reported to be a promising single imaging study for tumor staging, with few false-negative findings, whereas some false-positive findings have been reported for FDG PET (11,13). Inflammatory lesions, in particular, are known to accumulate FDG and pose a significant problem with using FDG in clinical oncology (34). In our study, 5 of 17 patients (29%) had more discrepant findings with FDG PET than with CT. Unfortunately, we could not histopathologically confirm all discrepant lesions.
Interestingly, the areas that were positive on PET but negative on CT almost coincided with both tracers. Not only FDG but also MET is known to accumulate in inflammatory tissue, although MET probably accumulates to a lesser extent (35–36). In the study of Yamada et al. (37), accumulation of MET in mediastinal and hilar lymph nodes in patients with active sarcoidosis was clearly shown.
CT showed enlarged lymph nodes in 4 areas with no FDG uptake and in 5 areas with no MET uptake. Half of these findings turned out to be from malignant causes and were seen in patients in whom enlarged para-aortic nodes disappeared as treatment began. The small size of these lesions was probably the major reason for negative findings. The central location together with lack of attenuation correction may have further affected lesion detectability in the para-aortic area. A general difference in the rate of lesion detection is unlikely, however, even if attenuation correction had been available in this study. Attenuation correction has not been shown to improve overall sensitivity for tumor detection either with conventional filtered backprojection reconstruction or new iterative reconstruction methods (38,39).The advantages of attenuation correction and sophisticated image reconstruction may manifest in easier image interpretation and better anatomic localization. Attenuation correction also makes possible the elimination of reconstruction artifacts and the quantification of tracer uptake. The full clinical impact of attenuation correction on staging lymphoma remains to be shown.
The diagnostic potential of CT still seems comparable in the abdomen, especially where avid uptake of tracer in the liver, pancreas, bone marrow, and intestines (MET) or urinary tract and intestines (FDG) interferes with interpretation of images. In clinical practice, the detection of lymphoma in small abdominal nodes may be a problem. Unfortunately, the current methodology for PET does not seem to resolve this challenge completely. Further clinical studies are warranted using improved techniques such as combined PET–CT imaging, which has recently been suggested to be a more useful diagnostic tool than PET alone in cancer patients (40). Overall, PET seems to show a greater number of positive lesions than does CT, although histologic confirmation of all lesions is usually lacking. A rigorous histologic verification of all suggestive findings in patients with lymphoma is, however, difficult because of possible multiorgan involvement in deeply situated sites.
CONCLUSION
Nodal staging of malignant lymphoma using WB PET with MET and FDG seems to be comparable with that using CT. Detection of nodal lymphoma with MET does not significantly differ from that with FDG. However, physiologic accumulations of MET may interfere with visual interpretation of PET images more than do physiologic accumulations of FDG, and hyperglycemic patients may be better imaged with MET than with FDG.
Acknowledgments
The authors thank Eeva Nordman and Juhani Knuuti for their valuable support and the personnel of the Turku PET Center, Department of Nuclear Medicine, and Department of Oncology and Radiotherapy of Turku University Central Hospital for their cooperation. This study was supported financially by the Finnish Cancer Society, Helsinki, Finland, and the Turku University Foundation, Turku, Finland.
Footnotes
Received Nov. 8, 1999; revision accepted May 5, 2000.
For correspondence or reprints contact: Eija Sutinen, MD, Department of Oncology and Radiotherapy, Turku University Central Hospital, P.O. Box 52, FIN-20521 Turku, Finland.